European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2018-01-02 , DOI: 10.1016/j.ejmech.2017.12.086 Xiao-san Li , Yi-chang Ren , Yu-zhou Bao , Jie Liu , Xiao-kun Zhang , You-wei Zhang , Xue-Long Sun , Xin-sheng Yao , Jin-Shan Tang
|
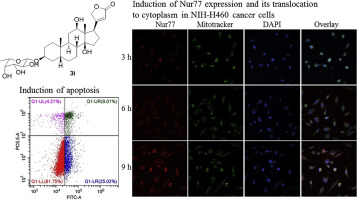
Cardiac glycosides exhibit significant anticancer effects and the glycosyl substitution at C3 position of digoxigenin is pivotal for their biological activity. In order to study the structure-activity relationship (SAR) of cardiac glycosides toward cancers and explore more potent anticancer agents, a series of C3-O-neoglycosides and C3-MeON-neoglycosides of digoxigenin were synthesized by the Koenigs-Knorr and neoglycosylation method, respectively. In addition, digoxigenin bisdigitoxoside and monodigitoxoside were prepared from digoxin by sodium periodate (NaIO4) oxidation and 6-aminocaproic acid hydrolysis. The SAR analysis revealed that C3-O-neoglycosides of digoxigenin exhibited stronger cytotoxicity and induction of Nur77 expression of tumor cells than C3-MeON-neoglycosides. Also, 3β-O-glycosides exhibited stronger anticancer effects than 3α-O-glycosides. Among them, 3β-O-(β-l-fucopyranosyl)-digoxigenin (3i) showed the highest activity on induction of Nur77 expression and translocation from the nucleus to cytoplasm, leading to cancer cell apoptosis.
中文翻译:

洋地黄毒苷的C 3-新糖苷的合成及其抗癌活性
心脏苷显示出显着的抗癌作用,洋地黄毒苷C 3位的糖基取代对其生物活性至关重要。为了研究强心苷的朝向癌症结构-活性关系(SAR)和探索更有效的抗癌剂,一系列的C 3 - ö -neoglycosides和C 3 -MeO Ñ -neoglycosides洋地黄毒苷的由合成的Koenigs-克诺尔和新糖基化方法。此外,地高辛通过高碘酸钠(NaIO 4)氧化和6-氨基己酸水解,从地高辛制备了洋地黄毒苷双指氧苷和单指氧苷。SAR分析表明C 3 - O洋地黄毒苷的β-新糖苷比C 3 -MeO N-新糖苷显示出更强的细胞毒性和对肿瘤细胞Nur77表达的诱导。另外,3 β - ö -glycosides显示比3更强的抗癌作用α - Ö -glycosides。其中,3 β - ø - (β -升-fucopyranosyl)-digoxigenin(3I)显示出对Nur77表达和转从细胞核至细胞质的诱导最高活性,导致癌细胞凋亡。


























 京公网安备 11010802027423号
京公网安备 11010802027423号