当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interactions of Dimethyltin(IV) with Uracil As Studied in the Gas Phase
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.jpca.7b11510 Jean-Yves Salpin 1, 2 , Latifa Latrous 3 , Violette Haldys 1, 2 , Al Mokhtar Lamsabhi
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.jpca.7b11510 Jean-Yves Salpin 1, 2 , Latifa Latrous 3 , Violette Haldys 1, 2 , Al Mokhtar Lamsabhi
Affiliation
|
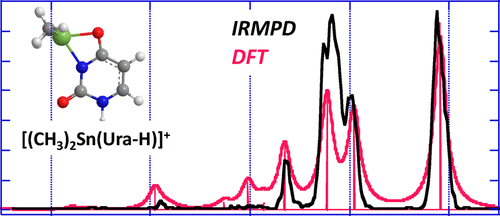
The gas-phase interactions of uracil (Ura) with dimethyltin(IV) were studied by a combined experimental and theoretical approach. Positive-ion electrospray spectra show that the interaction of dimethyltin(IV) with Ura results in the formation of the [(CH3)2Sn(Ura-H)]+ ion. The tandem mass spectrometry spectrum of this complex is characterized by numerous fragmentation processes, notably associated with elimination of H,N,C,O and C3,H3,N,O moieties, as well as the unusual loss of C2H6 leading to the [Sn(Ura-H)]+ complex. In turn, the [Sn(Ura-H)]+ complex fragments according to pathways already observed for the [Pb(Ura-H)]+ analogue. Sequential losses of ·CH3 radicals are also observed from the [(CH3)2Sn(N,C,O)]+ species (m/z 192). Comparison between density functional theory-computed vibrational spectra and the infrared multiple photon dissociation spectrum recorded between 1000 and 1900 cm–1 shows a good agreement as far as the global minimum is concerned. This comparison points to a bidentate interaction with a deprotonated canonical diketo form of uracil, involving both the N3 and O4 electronegative centers. This binding scheme has been already reported for the Pb/uracil system. The bidentate form characterized by the interaction between dimethyltin with N3 and O2 centers is slightly less stable. Interconversion between the two structures is associated with a small activation barrier (56 kJ/mol). The potential energy surfaces were explored to account for the main fragmentations observed upon collision-induced dissociation.
中文翻译:

气相研究二甲基锡(IV)与尿嘧啶的相互作用
通过实验和理论相结合的方法研究了尿嘧啶(Ura)与二甲基锡(IV)的气相相互作用。正离子电喷雾光谱表明,二甲基锡(IV)与Ura相互作用导致形成[(CH 3)2 Sn(Ura-H)] +离子。该络合物的串联质谱图的特征在于众多的碎片化过程,特别是与消除H,N,C,O和C 3,H 3,N,O部分以及异常的C 2 H 6损失有关。导致[Sn(Ura-H)] +络合物。反过来,[Sn(Ura-H)] +根据已经观察到的[Pb(Ura-H)] +类似物的途径选择复杂的片段。还从[(CH 3)2 Sn(N,C,O)] +物种(m / z 192)中观察到·CH 3自由基的顺序损失。密度泛函理论计算的振动光谱与记录在1000和1900 cm –1之间的红外多光子解离光谱之间的比较就全球最低要求而言,它显示出良好的协议。该比较表明与去质子化的经典二酮形式的尿嘧啶发生双齿相互作用,涉及N3和O4负电中心。对于Pb /尿嘧啶系统已经报道了这种结合方案。以二甲基锡与N3和O2中心之间的相互作用为特征的双齿形式的稳定性稍差。两种结构之间的相互转化与较小的活化势垒(56 kJ / mol)有关。探索了势能表面,以解释碰撞诱导的离解过程中观察到的主要碎裂现象。
更新日期:2018-01-17
中文翻译:

气相研究二甲基锡(IV)与尿嘧啶的相互作用
通过实验和理论相结合的方法研究了尿嘧啶(Ura)与二甲基锡(IV)的气相相互作用。正离子电喷雾光谱表明,二甲基锡(IV)与Ura相互作用导致形成[(CH 3)2 Sn(Ura-H)] +离子。该络合物的串联质谱图的特征在于众多的碎片化过程,特别是与消除H,N,C,O和C 3,H 3,N,O部分以及异常的C 2 H 6损失有关。导致[Sn(Ura-H)] +络合物。反过来,[Sn(Ura-H)] +根据已经观察到的[Pb(Ura-H)] +类似物的途径选择复杂的片段。还从[(CH 3)2 Sn(N,C,O)] +物种(m / z 192)中观察到·CH 3自由基的顺序损失。密度泛函理论计算的振动光谱与记录在1000和1900 cm –1之间的红外多光子解离光谱之间的比较就全球最低要求而言,它显示出良好的协议。该比较表明与去质子化的经典二酮形式的尿嘧啶发生双齿相互作用,涉及N3和O4负电中心。对于Pb /尿嘧啶系统已经报道了这种结合方案。以二甲基锡与N3和O2中心之间的相互作用为特征的双齿形式的稳定性稍差。两种结构之间的相互转化与较小的活化势垒(56 kJ / mol)有关。探索了势能表面,以解释碰撞诱导的离解过程中观察到的主要碎裂现象。

























 京公网安备 11010802027423号
京公网安备 11010802027423号