Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2018-01-01 , DOI: 10.1016/j.apcatb.2017.12.072 Douglas A.C. Coledam , Isaac Sánchez-Montes , Bianca F. Silva , José M. Aquino
|
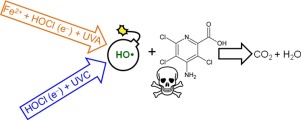
Four different treatment methods based on the HO production were assessed to oxidize and mineralize the herbicide picloram (PCL), which is considered very toxic and so is a potential contaminant of surface and ground water. The processes based on the Fenton type (homolysis reaction of HOCl by Fe2+ ions) and photo-Fenton type reaction (using a 9 W UVA light) with in situ electrogenerated HOCl species, using a commercial DSA® anode in the presence of Cl− ions, led to poor mineralization performances in comparison to the HOCl/UVC process. In that case, the homolysis reaction of HOCl mediated by a 5 or 9 W UVC light resulted in almost complete removal of the organic load within 12 h of treatment, from acidic to neutral solutions and using 1 g L−1 of NaCl concentration after optimization of the experimental conditions. When the HOCl/UVC process using a 5 W UVC light is compared to the electrochemical method using a boron-doped diamond anode (electrochemical/BDD), the oxidation and mineralization rates of the HOCl/UVC process were always superior, with ∼95% removal of total organic carbon (TOC) after 12 h treatment. The energy consumption per unit mass of removed TOC remained around 4 and 8 kW h g−1 for the HOCl/UVC and electrochemical/BDD treatment processes after 90% removal of TOC, respectively, even considering the energy consumption of the UVC lamp. In the final treatment stages, high CO2 conversions were obtained using both methods, as the generated intermediates were almost completely eliminated. Finally, the HOCl/UVC process is a reasonable option to treat solutions contaminated with organic pollutants as the common problems associated with the Fenton based (acidic solution, Fe2+ ion recovery, generation of H2O2) and electrochemical/BDD (mass transport) processes can be readily circumvented.
中文翻译:

用原位电生成活性氯矿化除草剂吡咯烷的HOCl / Fe 2 +,HOCl / Fe 2+ / UVA和HOCl / UVC工艺的性能
评估了基于HO生成量的四种不同处理方法,以氧化和矿化除草剂吡咯烷酮(PCL),后者被认为具有剧毒,因此也是地表水和地下水的潜在污染物。基于芬顿类型(用Fe次氯酸均裂反应的过程2+与离子)和光芬顿型反应(使用9瓦UVA光)在原位电致次氯酸物种,使用市售的DSA ®在氯的存在下阳极-与HOCl / UVC工艺相比,离子导致较差的矿化性能。在那种情况下,由5或9 W UVC光介导的HOCl的均相反应导致处理后12小时内从酸性溶液到中性溶液几乎完全去除有机负荷,并在优化后使用1 g L -1的NaCl浓度实验条件。当将使用5 W UVC光的HOCl / UVC工艺与使用掺硼金刚石阳极的电化学方法(electrochemical / BDD)进行比较时,HOCl / UVC工艺的氧化和矿化率始终较高,约为95%处理12小时后去除总有机碳(TOC)。去除的TOC的单位质量能耗保持在4 kWh g -1和8 kW h g -1左右去除90%的TOC后,分别用于HOCl / UVC和电化学/ BDD处理工艺,即使考虑到UVC灯的能耗也是如此。在最后的处理阶段,由于几乎完全消除了生成的中间体,因此使用这两种方法均获得了高的CO 2转化率。最后,HOCl / UVC工艺是处理被有机污染物污染的溶液的合理选择,这是与Fenton基(酸性溶液,Fe 2+离子回收,H 2 O 2的产生)和电化学/ BDD(质量)有关的常见问题。运输)流程可以很容易地规避。



























 京公网安备 11010802027423号
京公网安备 11010802027423号