当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comprehensive Analysis on the Highly Active and Stable PdAu/C Electrocatalyst for Ethanol Oxidation Reaction in Alkaline Media
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.jpcc.7b10009 Shuiyun Shen 1 , Yangge Guo 1 , Liuxuan Luo 1 , Fan Li 1 , Lin Li 1 , Guanghua Wei 2 , Jiewei Yin 1 , Changchun Ke 1 , Junliang Zhang 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.jpcc.7b10009 Shuiyun Shen 1 , Yangge Guo 1 , Liuxuan Luo 1 , Fan Li 1 , Lin Li 1 , Guanghua Wei 2 , Jiewei Yin 1 , Changchun Ke 1 , Junliang Zhang 1
Affiliation
|
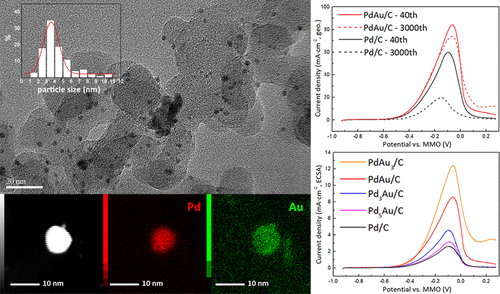
Both the catalytic activity and stability of palladium toward the ethanol oxidation reaction (EOR) in alkaline media are urgently required to be enhanced for the practical application of anion exchange membrane direct ethanol fuel cells (AEM DEFCs). The considerable improvement in the stability induced by the Au element has been well demonstrated; however, the catalytic activity from combining Pd with Au remains unsatisfied. Here, we successfully synthesize a series of PdxAuy/C electrocatalysts with different Pd/Au atomic ratios by a coreduction method, and in contrast to normal acknowledgments, the optimal PdAu/C sample not only possesses good stability but also has strikingly improved activity toward the EOR in alkaline media. Results show that the peak current density and degradation percentage for PdAu/C are, respectively, 84.16 mA/cm2 and 12% and 59.73 mA/cm2 and 67% for the pure Pd/C sample. It is anticipated that the striking activity toward the EOR can be attributed to an antipoisoning effect electronically induced by the Au addition, while the good stability can be ascribed to the formation of the stable Pd1Au1 alloy phase. It is also surprisingly revealed by physiochemical and electrochemical characterizations that there exists an unexpected monotone increasing trend for the promoting effect of Au content on Pd, and the compromise between the surface area specific activity and the number of active sites leads to the fact that the PdAu/C sample with Pd/Au atomic ratio of 1:1 has the highest mass activity toward the EOR in alkaline media.
中文翻译:

碱性介质中高活性稳定的PdAu / C电催化乙醇氧化反应的综合分析
对于阴离子交换膜直接乙醇燃料电池(AEM DEFCs)的实际应用,迫切需要提高钯在碱性介质中对乙醇氧化反应(EOR)的催化活性和稳定性。很好地证明了由金元素引起的稳定性的显着提高。但是,Pd与Au结合的催化活性仍然不令人满意。在这里,我们成功地合成了一系列Pd x Au yPd / Au原子比不同的/ C电催化剂通过共感应法得到的结果,与常规方法相比,最佳的PdAu / C样品不仅具有良好的稳定性,而且在碱性介质中具有显着提高的EOR活性。结果表明,纯Pd / C样品的PdAu / C峰值电流密度和降解百分数分别为84.16 mA / cm 2和12%,59.73 mA / cm 2和67%。预期对EOR的打击活性可以归因于Au添加电子诱导的抗毒作用,而良好的稳定性可以归因于稳定的Pd 1 Au 1的形成。合金相。物理化学和电化学特征也令人惊讶地揭示,存在着意想不到的单调增长趋势,以促进Au含量对Pd的促进作用,并且表面积比活性和活性位点数量之间的折衷导致了PdAu的事实。 Pd / Au原子比为1:1的/ C样品在碱性介质中对EOR具有最高的质量活性。
更新日期:2018-01-17
中文翻译:

碱性介质中高活性稳定的PdAu / C电催化乙醇氧化反应的综合分析
对于阴离子交换膜直接乙醇燃料电池(AEM DEFCs)的实际应用,迫切需要提高钯在碱性介质中对乙醇氧化反应(EOR)的催化活性和稳定性。很好地证明了由金元素引起的稳定性的显着提高。但是,Pd与Au结合的催化活性仍然不令人满意。在这里,我们成功地合成了一系列Pd x Au yPd / Au原子比不同的/ C电催化剂通过共感应法得到的结果,与常规方法相比,最佳的PdAu / C样品不仅具有良好的稳定性,而且在碱性介质中具有显着提高的EOR活性。结果表明,纯Pd / C样品的PdAu / C峰值电流密度和降解百分数分别为84.16 mA / cm 2和12%,59.73 mA / cm 2和67%。预期对EOR的打击活性可以归因于Au添加电子诱导的抗毒作用,而良好的稳定性可以归因于稳定的Pd 1 Au 1的形成。合金相。物理化学和电化学特征也令人惊讶地揭示,存在着意想不到的单调增长趋势,以促进Au含量对Pd的促进作用,并且表面积比活性和活性位点数量之间的折衷导致了PdAu的事实。 Pd / Au原子比为1:1的/ C样品在碱性介质中对EOR具有最高的质量活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号