当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemically Generated Recognition Sites in Self‐doped Polyaniline Modified Electrodes for Voltammetric and Potentiometric Determination of Copper(II) Ion
Electroanalysis ( IF 3 ) Pub Date : 2017-12-29 , DOI: 10.1002/elan.201700496 Saba Zamani Mohammadabadi 1 , Ali Reza Zanganeh 1
Electroanalysis ( IF 3 ) Pub Date : 2017-12-29 , DOI: 10.1002/elan.201700496 Saba Zamani Mohammadabadi 1 , Ali Reza Zanganeh 1
Affiliation
|
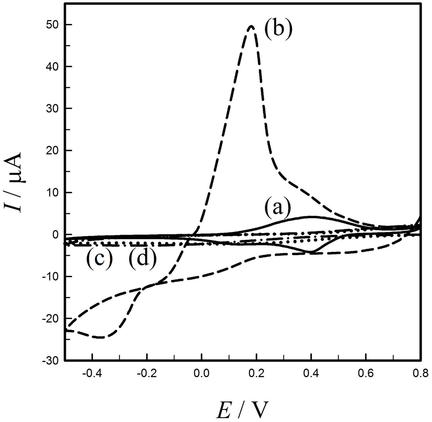
Chemical recognition elements for copper(II) ion have been generated in electrodes modified with poly(aniline‐co‐metanilic acid), P(An‐co‐MA), membrane and the resulting electrodes were used as selective sensors for voltammetric and potentiometric determination of this ion in an extended pH range. The P(An‐co‐MA) membrane was electrodeposited from aqueous mixed monomer solutions of An and MA, without the presence of a supporting electrolyte. For generating the recognition elements, P(An‐co‐MA) modified electrodes were subjected to several consecutive reduction/oxidation potential steps in copper(II) ion solution. It seems that during these potential steps, the receptor sites of the membrane are adjusted to the size, complexing property and hard/soft nature of copper(II) ion. This electrochemically mediated templating process, provided a selective sensor for determination of copper(II) ion. The results of preconcentration/differential pulse anodic stripping voltammetry, indicated analytical relation between the peak current and concentration of copper(II) from 1.0×10−9 to 1.0×10−4 M. The interference effect of various metal ions was explored and it was found that only mercury and silver ions show a considerable interference. The sensor exhibited selective potentiometric response for copper(II) over a wide concentration range (1.0×10−8 to 1.0×10−3 M) with a Nernstian slope of 27.9±0.3 mV per decade of copper(II) ion activity.
中文翻译:

自掺杂聚苯胺修饰电极中电化学产生的识别位点,用于伏安和电位测定法测定铜(II)离子
铜(II)离子的化学识别元素是在用聚苯胺-间-戊二酸,P(An-co-MA),膜修饰的电极中产生的,所得电极用作伏安法和电位测定法的选择性传感器在扩展的pH范围内 从An和MA的混合单体水溶液中电沉积P(An-co-MA)膜,不存在支持电解质。为了生成识别元素,P(An-co-MA)修饰电极在铜(II)离子溶液中经历了几个连续的还原/氧化电位步骤。似乎在这些潜在步骤中,将膜的受体位置调整为铜(II)离子的大小,络合特性和硬/软性质。这个电化学介导的模板过程 提供了一种用于测定铜(II)离子的选择性传感器。预浓缩/微分脉冲阳极溶出伏安法的结果表明,峰值电流与铜(II)的浓度之间的分析关系为1.0×10-9至1.0×10 -4M 。研究了各种金属离子的干扰作用,发现只有汞和银离子显示出相当大的干扰。该传感器在很宽的浓度范围(1.0×10 -8至1.0×10 -3 M)上表现出对铜(II)的选择性电位响应,每十年的铜(II)离子活度的Nernstian斜率为27.9±0.3 mV。
更新日期:2017-12-29
中文翻译:

自掺杂聚苯胺修饰电极中电化学产生的识别位点,用于伏安和电位测定法测定铜(II)离子
铜(II)离子的化学识别元素是在用聚苯胺-间-戊二酸,P(An-co-MA),膜修饰的电极中产生的,所得电极用作伏安法和电位测定法的选择性传感器在扩展的pH范围内 从An和MA的混合单体水溶液中电沉积P(An-co-MA)膜,不存在支持电解质。为了生成识别元素,P(An-co-MA)修饰电极在铜(II)离子溶液中经历了几个连续的还原/氧化电位步骤。似乎在这些潜在步骤中,将膜的受体位置调整为铜(II)离子的大小,络合特性和硬/软性质。这个电化学介导的模板过程 提供了一种用于测定铜(II)离子的选择性传感器。预浓缩/微分脉冲阳极溶出伏安法的结果表明,峰值电流与铜(II)的浓度之间的分析关系为1.0×10-9至1.0×10 -4M 。研究了各种金属离子的干扰作用,发现只有汞和银离子显示出相当大的干扰。该传感器在很宽的浓度范围(1.0×10 -8至1.0×10 -3 M)上表现出对铜(II)的选择性电位响应,每十年的铜(II)离子活度的Nernstian斜率为27.9±0.3 mV。



























 京公网安备 11010802027423号
京公网安备 11010802027423号