Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2017-12-29 , DOI: 10.1016/j.jinorgbio.2017.12.018 Daniel D. Turner , Latesh Lad , Hanna Kwon , Jaswir Basran , Katherine H. Carr , Peter C.E. Moody , Emma L. Raven
|
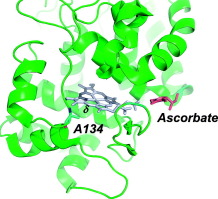
Ascorbate peroxidase (APX) is a class I heme peroxidase. It has two sites for binding of substrates. One is close to the γ-heme edge and is used for oxidation of ascorbate; the other is at the δ-heme edge and is used for binding of aromatic substrates [Gumiero et al., (2010) Arch. Biochem. Biophys. 500, 13–20]. In this work, we have examined the structural factors that control binding at the δ-heme edge by replacement of Ala134 in APX with a proline residue that is more commonly found in other class II and III peroxidases. Kinetic data indicate that replacement of Ala134 by proline has only a small effect on the catalytic mechanism, or the oxidation of ascorbate or guaiacol. Chemical modification with phenylhydrazine indicates that heme accessibility close to the δ-heme edge is only minorly affected by the substitution. We conclude that the A134P mutation alone is not enough to substantially affect the reactivity of APX towards aromatic substrates bound at the δ-heme edge. The data are relevant to the recent application of APX (APEX) in cellular imaging.
中文翻译:

Ala134在抗坏血酸过氧化物酶中控制底物结合和反应性的作用
抗坏血酸过氧化物酶(APX)是I类血红素过氧化物酶。它具有两个用于结合底物的位点。一种靠近γ-血红素边缘,用于抗坏血酸的氧化。另一个位于δ-血红素边缘,用于结合芳香族底物[Gumiero et al。,(2010)Arch。生化。生物物理学。500,13–20]。在这项工作中,我们研究了通过脯氨酸残基替代APX中的Ala134来控制δ-血红素边缘结合的结构因素,脯氨酸残基在其他II类和III类过氧化物酶中更常见。动力学数据表明,脯氨酸替代Ala134对催化机理或抗坏血酸或愈创木酚的氧化作用很小。用苯肼进行的化学修饰表明,接近δ-血红素边缘的血红素可及性仅受到取代的轻微影响。我们得出的结论是,仅A134P突变不足以实质性地影响APX对δ-血红素边缘结合的芳香族底物的反应性。数据与APX(APEX)在细胞成像中的最新应用有关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号