当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
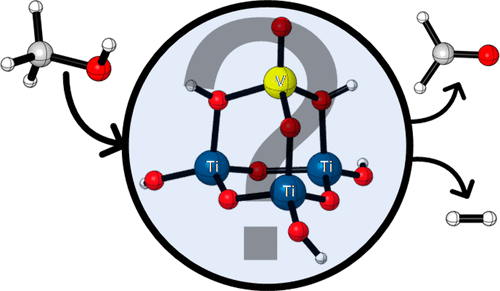
Can Supported Reduced Vanadium Oxides form H2 from CH3OH? A Computational Gas-Phase Mechanistic Study
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1021/acs.jpca.7b11264 Patricio González-Navarrete 1 , Juan Andrés 1 , Monica Calatayud 2, 3
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1021/acs.jpca.7b11264 Patricio González-Navarrete 1 , Juan Andrés 1 , Monica Calatayud 2, 3
Affiliation
|
A detailed density functional theory study is presented to clarify the mechanistic aspects of the methanol (CH3OH) dehydrogenation process to yield hydrogen (H2) and formaldehyde (CH2O). A gas-phase vanadium oxide cluster is used as a model system to represent reduced V(III) oxides supported on TiO2 catalyst. The theoretical results provide a complete scenario, involving several reaction pathways in which different methanol adsorption sites are considered, with presence of hydride and methoxide intermediates. Methanol dissociative adsorption process is both kinetically and thermodynamically feasible on V-O-Ti and V═O sites, and it might lead to form hydride species with interesting catalytic reactivity. The formation of H2 and CH2O on reduced vanadium sites, V(III), is found to be more favorable than for oxidized vanadium species, V(V), taking place along energy barriers of 29.9 and 41.0 kcal/mol, respectively.
中文翻译:

负载的还原钒氧化物能否从CH 3 OH形成H 2?计算气相机理研究
提出了详细的密度泛函理论研究,以阐明甲醇(CH 3 OH)脱氢过程产生氢(H 2)和甲醛(CH 2 O)的机理。气相钒氧化物簇用作模型系统,以表示负载在TiO 2催化剂上的还原V(III)氧化物。理论结果提供了一个完整的方案,涉及几种反应途径,其中考虑了不同的甲醇吸附位点,并存在氢化物和甲醇盐中间体。甲醇的解离吸附过程在VO-Ti和V = O位置上在动力学和热力学上都是可行的,并且可能导致形成具有有趣催化活性的氢化物。H的形成发现在还原的钒位点V(III)上的2和CH 2 O比氧化的钒物种V(V)分别沿29.9 kcal / mol和41.0 kcal / mol的能垒发生更有利。
更新日期:2018-01-19
中文翻译:

负载的还原钒氧化物能否从CH 3 OH形成H 2?计算气相机理研究
提出了详细的密度泛函理论研究,以阐明甲醇(CH 3 OH)脱氢过程产生氢(H 2)和甲醛(CH 2 O)的机理。气相钒氧化物簇用作模型系统,以表示负载在TiO 2催化剂上的还原V(III)氧化物。理论结果提供了一个完整的方案,涉及几种反应途径,其中考虑了不同的甲醇吸附位点,并存在氢化物和甲醇盐中间体。甲醇的解离吸附过程在VO-Ti和V = O位置上在动力学和热力学上都是可行的,并且可能导致形成具有有趣催化活性的氢化物。H的形成发现在还原的钒位点V(III)上的2和CH 2 O比氧化的钒物种V(V)分别沿29.9 kcal / mol和41.0 kcal / mol的能垒发生更有利。


























 京公网安备 11010802027423号
京公网安备 11010802027423号