当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Organocatalytic Asymmetric Vinylogous Aldol Reaction of Allyl Aryl Ketones to Silyl Glyoxylates
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.joc.7b02546 Man-Yi Han 1 , Wen-Yu Luan 1 , Pei-Lin Mai 1 , Pinhua Li 1 , Lei Wang 1, 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.joc.7b02546 Man-Yi Han 1 , Wen-Yu Luan 1 , Pei-Lin Mai 1 , Pinhua Li 1 , Lei Wang 1, 2
Affiliation
|
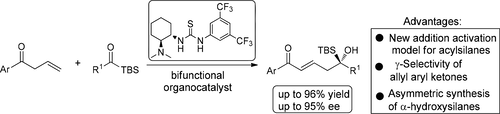
A direct organocatalytic asymmetric vinylogous aldol reaction of allyl aryl ketones to silyl glyoxylates has been developed through the bifunctional catalyst, giving the α-hydroxysilanes with excellent enantioselectivity (up to 95% ee) and in high yields (up to 96%). The success of this catalytic methodology offers an opportunity to tackle the problems in the nucleophilic addition to acylsilanes. To activate both allyl aryl ketones and acylsilanes, the utilized bifunctional catalyst was an ideal organocatalyst in this unprecedented transformation.
中文翻译:

烯丙基芳基酮与乙醛酸甲硅烷基酯的有机催化不对称乙烯基醛醇缩合反应
通过双功能催化剂开发了烯丙基芳基酮与乙醛酸甲硅烷基酯的直接有机催化不对称乙烯基醇醛缩醛反应,使α-羟基硅烷具有出色的对映选择性(高达95%ee)和高产率(高达96%)。这种催化方法的成功提供了解决酰基硅烷亲核加成中的问题的机会。为了活化烯丙基芳基酮和酰基硅烷,在这种空前的转化中,所用的双官能催化剂是理想的有机催化剂。
更新日期:2018-01-16
中文翻译:

烯丙基芳基酮与乙醛酸甲硅烷基酯的有机催化不对称乙烯基醛醇缩合反应
通过双功能催化剂开发了烯丙基芳基酮与乙醛酸甲硅烷基酯的直接有机催化不对称乙烯基醇醛缩醛反应,使α-羟基硅烷具有出色的对映选择性(高达95%ee)和高产率(高达96%)。这种催化方法的成功提供了解决酰基硅烷亲核加成中的问题的机会。为了活化烯丙基芳基酮和酰基硅烷,在这种空前的转化中,所用的双官能催化剂是理想的有机催化剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号