当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
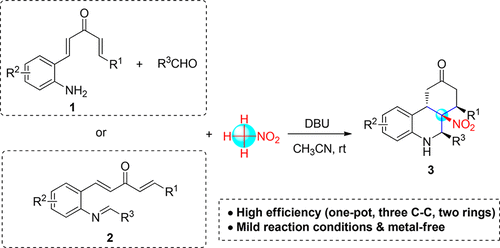
Triple Nucleophilic Attack of Nitromethane on (2-Iminoaryl)divinyl Ketones: A Domino Synthetic Strategy for Hexahydrophenanthridinones
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.joc.7b02759 Chengjie Feng 1 , Yifei Li 1 , Qi Xu 1 , Ling Pan 1 , Qun Liu 1 , Xianxiu Xu 1, 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.joc.7b02759 Chengjie Feng 1 , Yifei Li 1 , Qi Xu 1 , Ling Pan 1 , Qun Liu 1 , Xianxiu Xu 1, 2
Affiliation
|
A novel domino reaction of (2-iminoaryl)divinyl ketones with nitromethane was developed for the efficient synthesis of hexahydrophenanthridin-9(5H)-ones. The reaction proceeded smoothly from readily available starting materials under mild reaction conditions to construct three new bonds and two rings with high diastereoselectivities in good to excellent yields in a single step. A mechanism is proposed, involving a stepwise double Michael addition/aza-Henry reaction cascade, and in this transformation, nitromethane acts as a trinucleophile.
中文翻译:

硝基甲烷对(2-Iminoaryl)divinyl酮的三次亲核攻击:六氢菲基吡啶酮的多米诺合成策略
开发了一种新型的(2-亚氨基芳基)二乙烯基酮与硝基甲烷的多米诺反应,可有效合成六氢菲啶-9(5 H)-one。该反应在温和的反应条件下从容易获得的起始原料顺利进行,可在一个步骤中以良好或优异的收率构建出三个具有高非对映选择性的新键和两个环。提出了一种机理,涉及逐步的双迈克尔加成/氮杂-亨利反应级联,并且在该转化中,硝基甲烷充当三亲核试剂。
更新日期:2018-01-16
中文翻译:

硝基甲烷对(2-Iminoaryl)divinyl酮的三次亲核攻击:六氢菲基吡啶酮的多米诺合成策略
开发了一种新型的(2-亚氨基芳基)二乙烯基酮与硝基甲烷的多米诺反应,可有效合成六氢菲啶-9(5 H)-one。该反应在温和的反应条件下从容易获得的起始原料顺利进行,可在一个步骤中以良好或优异的收率构建出三个具有高非对映选择性的新键和两个环。提出了一种机理,涉及逐步的双迈克尔加成/氮杂-亨利反应级联,并且在该转化中,硝基甲烷充当三亲核试剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号