当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Organocatalytic Synthesis of 4-Aryl-1,2,3,4-tetrahydropyridines from Morita-Baylis-Hillman Carbonates through a One-Pot Three-Component Cyclization
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.joc.7b02818 Jian Wei 1 , Yuntong Li 1 , Cheng Tao 1 , Huifei Wang 2 , Bin Cheng 1 , Hongbin Zhai 2 , Yun Li 1, 3
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.joc.7b02818 Jian Wei 1 , Yuntong Li 1 , Cheng Tao 1 , Huifei Wang 2 , Bin Cheng 1 , Hongbin Zhai 2 , Yun Li 1, 3
Affiliation
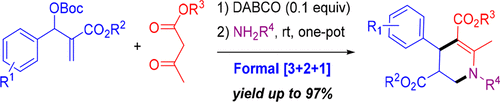
|
An efficient DABCO-catalyzed three-component formal [3 + 2 + 1] annulation, involving a Morita-Baylis-Hillman carbonate, a 1,3-ketoester, and a primary amine, leading to one-pot synthesis of substituted 4-aryl-1,2,3,4-tetrahydropyridines, has been developed. The densely functionalized products were generally obtained in good to excellent yields under mild conditions. The structures including the relative stereoconfigurations of the representative products were confirmed by X-ray diffraction analysis.
中文翻译:

一锅三组分环化反应由森田-贝利斯-希尔曼碳酸盐有机催化合成4-芳基1,2,3,4-四氢吡啶
一种有效的DABCO催化的三组分正式[3 + 2 +1]环化反应,涉及Morita-Baylis-Hillman碳酸盐,1,3-酮酸酯和伯胺,导致一锅合成取代的4-芳基-1,2,3,4-四氢吡啶已被开发出来。通常在温和的条件下以高至优异的产率获得致密官能化的产物。通过X射线衍射分析确认了包括代表产物的相对立体构型的结构。
更新日期:2018-01-10
中文翻译:

一锅三组分环化反应由森田-贝利斯-希尔曼碳酸盐有机催化合成4-芳基1,2,3,4-四氢吡啶
一种有效的DABCO催化的三组分正式[3 + 2 +1]环化反应,涉及Morita-Baylis-Hillman碳酸盐,1,3-酮酸酯和伯胺,导致一锅合成取代的4-芳基-1,2,3,4-四氢吡啶已被开发出来。通常在温和的条件下以高至优异的产率获得致密官能化的产物。通过X射线衍射分析确认了包括代表产物的相对立体构型的结构。



























 京公网安备 11010802027423号
京公网安备 11010802027423号