European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2017-12-30 , DOI: 10.1016/j.ejmech.2017.12.085 Sundus Akhter , Bjarte Aarmo Lund , Aya Ismael , Manuel Langer , Johan Isaksson , Tony Christopeit , Hanna-Kirsti S. Leiros , Annette Bayer
|
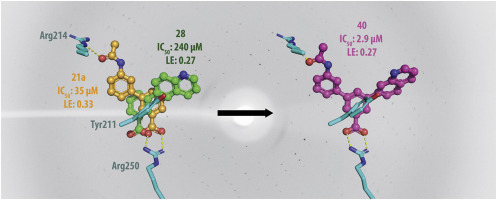
β-Lactam antibiotics are of utmost importance when treating bacterial infections in the medical community. However, currently their utility is threatened by the emergence and spread of β-lactam resistance. The most prevalent resistance mechanism to β-lactam antibiotics is expression of β-lactamase enzymes. One way to overcome resistance caused by β-lactamases, is the development of β-lactamase inhibitors and today several β-lactamase inhibitors e.g. avibactam, are approved in the clinic. Our focus is the oxacillinase-48 (OXA-48), an enzyme reported to spread rapidly across the world and commonly identified in Escherichia coli and Klebsiella pneumoniae. To guide inhibitor design, we used diversely substituted 3-aryl and 3-heteroaryl benzoic acids to probe the active site of OXA-48 for useful enzyme-inhibitor interactions. In the presented study, a focused fragment library containing 49 3-substituted benzoic acid derivatives were synthesised and biochemically characterized. Based on crystallographic data from 33 fragment-enzyme complexes, the fragments could be classified into R1 or R2 binders by their overall binding conformation in relation to the binding of the R1 and R2 side groups of imipenem. Moreover, binding interactions attractive for future inhibitor design were found and their usefulness explored by the rational design and evaluation of merged inhibitors from orthogonally binding fragments. The best inhibitors among the resulting 3,5-disubstituted benzoic acids showed inhibitory potential in the low micromolar range (IC50 = 2.9 μM). For these inhibitors, the complex X-ray structures revealed non-covalent binding to Arg250, Arg214 and Tyr211 in the active site and the interactions observed with the mono-substituted fragments were also identified in the merged structures.
中文翻译:

针对抗生素抗性酶-Oxacillinase-48的聚焦片段文库:合成,结构评估和抑制剂设计
当在医学界中治疗细菌感染时,β-内酰胺抗生素至关重要。然而,目前它们的效用受到β-内酰胺抗性的出现和扩散的威胁。对β-内酰胺抗生素最普遍的耐药机制是β-内酰胺酶的表达。克服由β-内酰胺酶引起的抗性的一种方法是开发β-内酰胺酶抑制剂,如今临床上已经批准了几种β-内酰胺酶抑制剂,例如阿维巴坦。我们的研究重点是oxacillinase-48(OXA-48),据报道该酶在世界范围内迅速传播,通常在大肠杆菌和肺炎克雷伯菌中得到鉴定。为了指导抑制剂的设计,我们使用了不同取代的3-芳基和3-杂芳基苯甲酸来探测OXA-48的活性位点,以进行有用的酶-抑制剂相互作用。在本研究中,合成了包含49个3-取代的苯甲酸衍生物的聚焦片段文库,并对其进行了生化表征。基于从33片段的酶复合物晶体学数据,片段可以被归类为R 1或R 2个可以通过总体结合构象相对于粘合剂到R的结合1和R 2亚胺培南的侧基。此外,发现了对未来抑制剂设计具有吸引力的结合相互作用,并通过合理设计和评估来自正交结合片段的合并抑制剂来探索其有用性。在所得的3,5-二取代的苯甲酸中,最好的抑制剂在低微摩尔范围内(IC 50 = 2.9μM)表现出抑制潜力。对于这些抑制剂,复杂的X射线结构揭示了活性位点与Arg250,Arg214和Tyr211的非共价结合,并且在合并的结构中还鉴定出与单取代片段的相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号