Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2017-12-29 , DOI: 10.1016/j.bmcl.2017.12.064 Jianbo Wu , Chunkai Wang , Derek Leas , Mireille Vargas , Karen L. White , David M. Shackleford , Gong Chen , Austin G. Sanford , Ryan M. Hemsley , Paul H. Davis , Yuxiang Dong , Susan A. Charman , Jennifer Keiser , Jonathan L. Vennerstrom
|
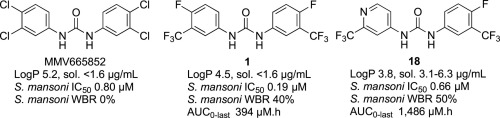
N,N′-Diaryl ureas have recently emerged as a new antischistosomal chemotype. We now describe physicochemical profiling, in vitro ADME, plasma exposure, and ex vivo and in vivo activities against Schistosoma mansoni for twenty new N,N′-diaryl ureas designed primarily to increase aqueous solubility, but also to maximize structural diversity. Replacement of one of the 4-fluoro-3-trifluoromethylphenyl substructures of lead N,N′-diaryl urea 1 with azaheterocycles and benzoic acids, benzamides, or benzonitriles decreased lipophilicity, and in most cases, increased aqueous solubility. There was no clear relationship between lipophilicity and metabolic stability, although all compounds with 3-trifluoromethyl-4-pyridyl substructures were metabolically stable. N,N′-diaryl ureas containing 4-fluoro-3-trifluoromethylphenyl, 3-trifluoromethyl-4-pyridyl, 2,2-difluorobenzodioxole, or 4-benzonitrile substructures had high activity against ex vivo S. mansoni and relatively low cytotoxicity. N,N-diaryl ureas with 3-trifluoromethyl-4-pyridyl and 2,2-difluorobenzodioxole substructures had the highest exposures whereas those with 4-fluoro-3-trifluoromethylphenyl substructures had the best in vivo antischistosomal activities. There was no direct correlation between compound exposure and in vivo activity.
中文翻译:

抗血吸虫性N,N'-二芳基脲SAR的研究进展。
N,N′-二芳基尿素最近已经出现为新的抗血吸虫化学型。现在,我们描述了针对曼氏血吸虫的二十种新的N,N'-二芳基尿素的理化概况分析,体外ADME,血浆暴露以及离体和体内活性,这些N,N'-二芳基尿素主要设计用于增加水溶性,同时也可以最大程度地提高结构多样性。N,N'-二芳基尿素1铅的4-氟-3-三氟甲基苯基亚结构之一的取代与氮杂杂环和苯甲酸,苯甲酰胺或苄腈一起使用会降低亲脂性,并且在大多数情况下会增加水溶性。亲脂性与代谢稳定性之间没有明确的关系,尽管所有具有3-三氟甲基-4-吡啶基亚结构的化合物在代谢上都是稳定的。含有4-氟-3-三氟甲基苯基,3-三氟甲基-4-吡啶基,2,2-二氟苯并二恶唑或4-苄腈亚结构的N,N′-二芳基脲对离体曼氏梭菌具有高活性,并且细胞毒性相对较低。ñ,ñ具有3-三氟甲基-4-吡啶基和2,2-二氟苯并二恶唑亚结构的-二芳基尿素具有最高的暴露量,而具有4-氟-3-三氟甲基苯基亚结构的那些具有最佳的体内抗血吸虫病活性。化合物暴露与体内活性之间没有直接关系。


























 京公网安备 11010802027423号
京公网安备 11010802027423号