Tetrahedron ( IF 2.1 ) Pub Date : 2017-12-29 , DOI: 10.1016/j.tet.2017.12.051 Peili Sun , Danling Rao , Pu Zhang , Yujun Qin , Zhi-Xin Guo
|
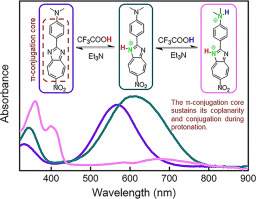
Three 2-phenyl-1,3-diazaazulene derivatives were synthesized and their protonation behaviours were investigated systematically via UV–vis absorption titration and 1H NMR titration, as well as theoretical calculations. One of them exhibited a monoprotonation process while the others displayed prominent halochromic diprotonation responses. Interestingly, upon protonation of 2-phenyl-1,3-diazaazulene derivatives, the coplanarity and conjugation of the 16-π-conjugated backbones were well kept, while the electronic structures were controllably adjusted. The response mechanism of 1,3-diazaazulene derivatives towards acid is through the attachment of acid proton to the nitrogen atom in the diazaazulene ring, resulting in the change of the hybridization of protonated-N from sp2 to sp3, which differed from that of the well-known azulene (analogue of 1,3-diazaazulene, protonation at carbon atom). This work would provide a new insight into the protonation research of the organic functional molecules.
中文翻译:

2-苯基-1,3-二氮杂氮烯衍生物的质子化行为
合成了三个2-苯基-1,3-二氮杂氮烯衍生物,并通过紫外可见吸收滴定和1 H NMR滴定以及理论计算系统地研究了它们的质子化行为。其中一个表现出单质子化过程,而其他表现出显着的卤化双质子化反应。有趣的是,在对2-苯基-1,3-二氮杂氮烯衍生物进行质子化时,可以很好地保持16-π共轭骨架的共面性和共轭性,同时可控地调节电子结构。1,3-二氮杂氮烯衍生物对酸的反应机理是通过酸质子与二氮杂氮烯环中氮原子的连接,导致质子化的N与sp 2的杂交发生改变。到sp 3,不同于众所周知的a(1,3-二氮杂az的类似物,在碳原子上质子化)。这项工作将为有机功能分子的质子化研究提供新的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号