Tetrahedron ( IF 2.1 ) Pub Date : 2017-12-29 , DOI: 10.1016/j.tet.2017.12.054 Kazuaki Kuwata , Rie Fujita , Kengo Hanaya , Shuhei Higashibayashi , Takeshi Sugai
|
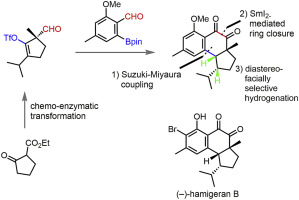
A formal total synthesis of (−)-hamigeran B was achieved in 17 steps from commercially available ethyl 2-oxocyclopentanecarboxylate. Carbonyl reductase-catalyzed asymmetric reduction and the subsequent chemical transformations furnished an enantiomerically pure synthetic intermediate, (R)-5-formyl-2-isopropyl-5-methylcyclopent-1-en-1-yl trifluoromethylsulfonate. Suzuki-Miyaura coupling with Gao's arylboronate [2-(2-formyl-3-methoxy-5-methylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane], under PdCl2(dppf)•CH2Cl2 catalysis, and the subsequent cyclization by way of intramolecular reductive SmI2-mediated 1,2-diol formation provided a tricyclic skeleton with a tetrasubstituted double bond between C-1 and C-9b. Upon hydrogenation of this double bond, the proper stereochemistry of the remaining chiral centers was established. Exclusive addition of the hydrogen atom from the β-face occurred, owing to the shielding of the α-face with a bulky TBS protective group on the C-4 alcohol. The hydrogenation products were transformed into Clive's synthetic precursor for (−)-hamigeran B.
中文翻译:

从化学酶法制备的具有季手性中心的结构单元中正式合成(-)-Hamigeran B
(-)-hamigeran B的正式全合成是通过17个步骤从市售的2-氧代环戊烷甲酸乙酯实现的。羰基还原酶催化的不对称还原和随后的化学转化提供了对映体纯的合成中间体,(R)-5-甲酰基-2-异丙基-5-甲基环戊-1-烯-1-基三氟甲基磺酸酯。在PdCl 2(dppf)下,Suzuki-Miyaura与Gao的芳基硼酸酯[2-(2-甲酰基-3-甲氧基-5-甲基苯基)-4,4,5,5-四甲基-1,3,2-二氧杂硼烷]偶联• CH 2 Cl 2催化,以及随后通过分子内还原性SmI 2的环化介导的1,2-二醇的形成提供了在C-1和C-9b之间具有四取代的双键的三环骨架。该双键氢化后,建立了剩余手性中心的适当立体化学。由于α面被C-4醇上的庞大的TBS保护基所屏蔽,因此发生了β面氢原子的排他性加成反应。氢化产物被转化为Clive的(-)-hamigeran B的合成前体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号