当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design of an Activity-Based Probe for Human Neutrophil Elastase: Implementation of the Lossen Rearrangement To Induce Förster Resonance Energy Transfers
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.biochem.7b00906 Anna-Christina Schulz-Fincke 1 , Alexander S. Tikhomirov 1, 2 , Annett Braune 3 , Tamara Girbl 4 , Erik Gilberg 1, 5 , Jürgen Bajorath 5 , Michael Blaut 3 , Sussan Nourshargh 4 , Michael Gütschow 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.biochem.7b00906 Anna-Christina Schulz-Fincke 1 , Alexander S. Tikhomirov 1, 2 , Annett Braune 3 , Tamara Girbl 4 , Erik Gilberg 1, 5 , Jürgen Bajorath 5 , Michael Blaut 3 , Sussan Nourshargh 4 , Michael Gütschow 1
Affiliation
|
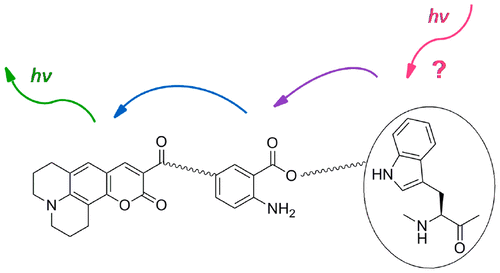
Human neutrophil elastase is an important regulator of the immune response and plays a role in host defense mechanisms and further physiological processes. The uncontrolled activity of this serine protease may cause severe tissue alterations and impair inflammatory states. The design of an activity-based probe for human neutrophil elastase reported herein relies on a sulfonyloxyphthalimide moiety as a new type of warhead that is linker-connected to a coumarin fluorophore. The inhibitory potency of the activity-based probe was assessed against several serine and cysteine proteases, and the selectivity for human neutrophil elastase (Ki = 6.85 nM) was determined. The adequate fluorescent tag of the probe allowed for the in-gel fluorescence detection of human neutrophil elastase in the low nanomolar range. The coumarin moiety and the anthranilic acid function of the probe, produced in the course of a Lossen rearrangement, were part of two different Förster resonance energy transfers.
中文翻译:

基于活动的人类嗜中性粒细胞弹性蛋白酶探针的设计:丢失重排的实现以诱导福斯特共振能量转移。
人嗜中性粒细胞弹性蛋白酶是免疫反应的重要调节剂,并在宿主防御机制和进一步的生理过程中起作用。这种丝氨酸蛋白酶的不受控制的活性可能导致严重的组织改变并损害炎症状态。本文报道的基于人中性粒细胞弹性蛋白酶的基于活性的探针的设计依赖于磺酰氧基邻苯二甲酰亚胺部分作为连接至香豆素荧光团的新型战斗部。评估了基于活性的探针对几种丝氨酸和半胱氨酸蛋白酶的抑制能力,以及对人嗜中性粒细胞弹性蛋白酶的选择性(K i= 6.85 nM)。探针的适当荧光标签可在低纳摩尔范围内进行人嗜中性粒细胞弹性蛋白酶的凝胶内荧光检测。在Lossen重排过程中产生的探针的香豆素部分和邻氨基苯甲酸功能是两种不同的Förster共振能量转移的一部分。
更新日期:2018-01-16
中文翻译:

基于活动的人类嗜中性粒细胞弹性蛋白酶探针的设计:丢失重排的实现以诱导福斯特共振能量转移。
人嗜中性粒细胞弹性蛋白酶是免疫反应的重要调节剂,并在宿主防御机制和进一步的生理过程中起作用。这种丝氨酸蛋白酶的不受控制的活性可能导致严重的组织改变并损害炎症状态。本文报道的基于人中性粒细胞弹性蛋白酶的基于活性的探针的设计依赖于磺酰氧基邻苯二甲酰亚胺部分作为连接至香豆素荧光团的新型战斗部。评估了基于活性的探针对几种丝氨酸和半胱氨酸蛋白酶的抑制能力,以及对人嗜中性粒细胞弹性蛋白酶的选择性(K i= 6.85 nM)。探针的适当荧光标签可在低纳摩尔范围内进行人嗜中性粒细胞弹性蛋白酶的凝胶内荧光检测。在Lossen重排过程中产生的探针的香豆素部分和邻氨基苯甲酸功能是两种不同的Förster共振能量转移的一部分。


























 京公网安备 11010802027423号
京公网安备 11010802027423号