当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Formal Total Syntheses of (−)- and (+)-Actinophyllic Acid
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.joc.7b02747 Fei Xue 1 , Huifang Lu 1 , Liping He 1 , Wenfei Li 1 , Dan Zhang 1 , Xiao-Yu Liu 1 , Yong Qin 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.joc.7b02747 Fei Xue 1 , Huifang Lu 1 , Liping He 1 , Wenfei Li 1 , Dan Zhang 1 , Xiao-Yu Liu 1 , Yong Qin 1
Affiliation
|
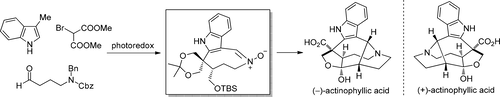
The formal total syntheses of (−)-actinophyllic acid and its enantiomer starting from the same chiral intermediate are reported. The synthesis features a photoredox organocatalytic asymmetric alkylation to generate the original C15 chirality, a photocatalytic C–H functionalization of 3-methylindole in flow for constructing the C16 all-carbon quaternary center, a regioselective 1,3-dipolar cycloaddition, and an intramolecular Henry reaction to assemble the pentacyclic core of the target molecule.
中文翻译:

(-)-和(+)-猕猴桃酸的形式总合成
据报道,从相同的手性中间体开始,(-)-放线菌酸及其对映异构体的正式全部合成。该合成具有光氧化还原有机催化不对称烷基化以产生原始的C15手性,3-甲基吲哚的光催化C–H官能化以构建C16全碳四元中心,区域选择性的1,3-偶极环加成和分子内亨利反应以组装靶分子的五环核。
更新日期:2018-01-10
中文翻译:

(-)-和(+)-猕猴桃酸的形式总合成
据报道,从相同的手性中间体开始,(-)-放线菌酸及其对映异构体的正式全部合成。该合成具有光氧化还原有机催化不对称烷基化以产生原始的C15手性,3-甲基吲哚的光催化C–H官能化以构建C16全碳四元中心,区域选择性的1,3-偶极环加成和分子内亨利反应以组装靶分子的五环核。



























 京公网安备 11010802027423号
京公网安备 11010802027423号