当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Double-Modified Xyloside Analogues for Probing the β4GalT7 Active Site
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.joc.7b02809 Daniel Willén 1 , Dennis Bengtsson 1 , Sebastian Clementson 1 , Emil Tykesson 1 , Sophie Manner 1 , Ulf Ellervik 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.joc.7b02809 Daniel Willén 1 , Dennis Bengtsson 1 , Sebastian Clementson 1 , Emil Tykesson 1 , Sophie Manner 1 , Ulf Ellervik 1
Affiliation
|
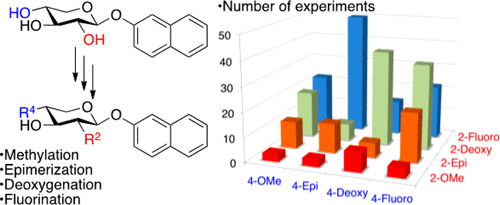
Monosubstituted naphthoxylosides have been shown to function as substrates for, and inhibitors of, the enzyme β4GalT7, a key enzyme in the biosynthetic pathway leading to glycosaminoglycans and proteoglycans. In this article, we explore the synthesis of 16 xyloside analogues, modified at two different positions, as well as their function as inhibitors of and/or substrates for the enzyme. Seemingly simple compounds turned out to require complex synthetic pathways. A meta-analysis of the synthetic work shows that, regardless of the abundance of methods available for carbohydrate synthesis, even simple modifications can turn out to be problematic, and double modifications present additional challenges due to conformational, steric, and stereoelectronic effects.
中文翻译:

双修饰木糖苷类似物的合成以探测β4GalT7活性位点
已经显示单取代的萘氧基氯化物作为酶β4GalT7的底物和抑制剂,β4GalT7是导致糖胺聚糖和蛋白聚糖的生物合成途径中的关键酶。在本文中,我们探索了在两个不同位置修饰的16种木糖苷类似物的合成,以及它们作为酶的抑制剂和/或底物的功能。看来简单的化合物需要复杂的合成途径。对合成工作的荟萃分析表明,不管碳水化合物合成方法的丰富性如何,即使简单的修饰也可能是有问题的,而双重修饰由于构象,空间和立体电子效应而带来了其他挑战。
更新日期:2018-01-12
中文翻译:

双修饰木糖苷类似物的合成以探测β4GalT7活性位点
已经显示单取代的萘氧基氯化物作为酶β4GalT7的底物和抑制剂,β4GalT7是导致糖胺聚糖和蛋白聚糖的生物合成途径中的关键酶。在本文中,我们探索了在两个不同位置修饰的16种木糖苷类似物的合成,以及它们作为酶的抑制剂和/或底物的功能。看来简单的化合物需要复杂的合成途径。对合成工作的荟萃分析表明,不管碳水化合物合成方法的丰富性如何,即使简单的修饰也可能是有问题的,而双重修饰由于构象,空间和立体电子效应而带来了其他挑战。



























 京公网安备 11010802027423号
京公网安备 11010802027423号