当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phase Transition of FeSO4·7H2O to FeSO4·H2O in the H2SO4–HCl–H2O System by Modeling Solubility
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acssuschemeng.7b03627 Juan Zhou 1, 2 , Yan Zeng 3 , George P. Demopoulos 3 , Chunxi Li 1 , Zhibao Li 2
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2018-01-18 00:00:00 , DOI: 10.1021/acssuschemeng.7b03627 Juan Zhou 1, 2 , Yan Zeng 3 , George P. Demopoulos 3 , Chunxi Li 1 , Zhibao Li 2
Affiliation
|
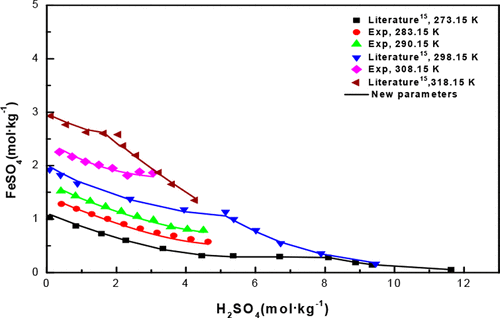
A new sustainable process was developed to recycle FeSO4·7H2O produced from the sulfate process for titanium dioxide production. In this process, FeSO4·H2O was crystallized out from a subsequent cooling crystallization operation rather than FeSO4·7H2O. In order to better develop the new process and provide a theoretical basis for ferrous sulfate dehydration, the study of the phase equilibrium of the FeSO4–H2SO4–HCl–H2O system is necessary. For this purpose, the solubility of FeSO4·7H2O in the H2SO4–HCl–H2O system was determined at a temperature range from 278.15 to 313.15 K using a dynamic method. By raising temperature and the concentration of HCl, more FeSO4·7H2O can be dissolved in this system. However, with the addition of H2SO4, the solubility of FeSO4·7H2O is found to decrease due to common-ion effect. A speciation-based model equipped with middle-range parameters was established by regressing the experimental and reported solubility of FeSO4·7H2O under varying conditions. The phase transition temperature of FeSO4·7H2O to FeSO4·H2O was successfully predicted by the new model, which was found to decrease with increasing concentration of both H2SO4 and HCl.
中文翻译:

H 2 SO 4 -HCl–H 2 O体系中FeSO 4 ·7H 2 O到FeSO 4 ·H 2 O的相变建模
一个新的过程可持续的开发是为了再循环的FeSO 4 ·7H 2从二氧化钛生产硫酸盐法产生的O操作。在这个过程中,的FeSO 4 ·H 2 O的从后续冷却结晶操作结晶出来,而不是的FeSO 4 ·7H 2 O.为了更好地开发新进程和硫酸亚铁脱水提供理论基础,的研究FeSO 4 –H 2 SO 4 –HCl–H 2 O系统的相平衡是必要的。为此目的,FeSO 4 ·7H 2 O在H 2 SO中的溶解度使用动态方法在278.15至313.15 K的温度范围内测定了4 –HCl–H 2 O系统。通过提高温度和HCl的浓度,更多的FeSO 4 ·7H 2 O可以溶解在该系统中。然而,发现由于添加了H 2 SO 4,FeSO 4 ·7H 2 O的溶解度由于共离子效应而降低。通过回归实验和报道的FeSO 4 ·7H 2 O在不同条件下的溶解度,建立了带有中间范围参数的基于物种的模型。FeSO 4 ·7H 2 O转变为FeSO的相变温度新模型成功预测了4 ·H 2 O,发现随着H 2 SO 4和HCl浓度的增加,H 2 O会降低。
更新日期:2018-01-18
中文翻译:

H 2 SO 4 -HCl–H 2 O体系中FeSO 4 ·7H 2 O到FeSO 4 ·H 2 O的相变建模
一个新的过程可持续的开发是为了再循环的FeSO 4 ·7H 2从二氧化钛生产硫酸盐法产生的O操作。在这个过程中,的FeSO 4 ·H 2 O的从后续冷却结晶操作结晶出来,而不是的FeSO 4 ·7H 2 O.为了更好地开发新进程和硫酸亚铁脱水提供理论基础,的研究FeSO 4 –H 2 SO 4 –HCl–H 2 O系统的相平衡是必要的。为此目的,FeSO 4 ·7H 2 O在H 2 SO中的溶解度使用动态方法在278.15至313.15 K的温度范围内测定了4 –HCl–H 2 O系统。通过提高温度和HCl的浓度,更多的FeSO 4 ·7H 2 O可以溶解在该系统中。然而,发现由于添加了H 2 SO 4,FeSO 4 ·7H 2 O的溶解度由于共离子效应而降低。通过回归实验和报道的FeSO 4 ·7H 2 O在不同条件下的溶解度,建立了带有中间范围参数的基于物种的模型。FeSO 4 ·7H 2 O转变为FeSO的相变温度新模型成功预测了4 ·H 2 O,发现随着H 2 SO 4和HCl浓度的增加,H 2 O会降低。



























 京公网安备 11010802027423号
京公网安备 11010802027423号