当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In Vitro and in Silico Competitive Binding of Brominated Polyphenyl Ether Contaminants with Human and Gull Thyroid Hormone Transport Proteins
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.est.7b04617 Katie L. Hill 1, 2, 3 , Åse-Karen Mortensen 4 , Daniel Teclechiel 5 , William G. Willmore 2 , Ingebrigt Sylte 6 , Bjørn M. Jenssen 4 , Robert J. Letcher 1, 2
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.est.7b04617 Katie L. Hill 1, 2, 3 , Åse-Karen Mortensen 4 , Daniel Teclechiel 5 , William G. Willmore 2 , Ingebrigt Sylte 6 , Bjørn M. Jenssen 4 , Robert J. Letcher 1, 2
Affiliation
|
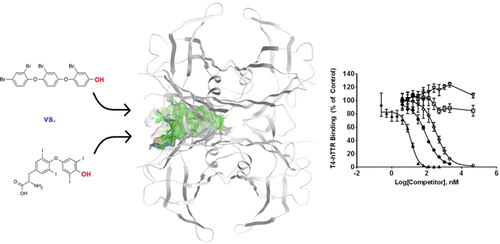
Tetradecabromo-1,4-diphenoxybenzene (TeDB-DiPhOBz) is a highly brominated additive flame retardant (FR). Debrominated photodegradates of TeDB-DiPhOBz are hydroxylated in vitro in liver microsomal assays based on herring gulls (Larus argentatus), including one metabolite identified as 4″-OH-2,2′,2″,4-tetrabromo-DiPhOBz. Chemically related methoxylated tetra- to hexabromo-DiPhOBzs are known contaminants in herring gulls. Collectively, nothing is currently known about biological effects of these polybrominated (PB) DiPhOBz-based compounds. The present study investigated the potential thyroidogenicity of 2,2′,2″,4-tetrabromo-(TB)-DiPhOBz along with its para-methoxy (MeO)- and hydroxy-(OH)-analogues, using an in vitro competitive protein binding assay with the human thyroid hormone (TH) transport proteins transthyretin (hTTR) and albumin (hALB). This model para-OH-TB-DiPhOBz was found to be capable of competing with thyroxine (T4) for the binding site on hTTR and hALB. In silico analyses were also conducted using a 3D homology model for gull TTR, to predict whether these TB-DiPhOBz-based compounds may also act as ligands for an avian TH transport protein despite evolutionary differences with hTTR. This analysis found all three TB-DiPhOBz analogues to be potential ligands for gull TTR and have similar binding efficacies to THs. Results indicate structure-related differences in binding affinities of these ligands and suggest there is potential for these contaminants to interact with both mammalian and avian thyroid function.
中文翻译:

溴化聚苯醚污染物与人和鸥甲状腺激素转运蛋白的体外和计算机竞争性结合
Tetradecabromo-1,4-diphenoxybenzene(TeDB-DiPhOBz)是一种高度溴化的添加剂阻燃剂(FR)。在基于鲱鸥(Larus argentatus)的肝微粒体测定法中,将TeDB-DiPhOBz的脱溴光降解物在体外进行羟基化,包括一种被鉴定为4” -OH-2,2',2”,4-四溴-DiPhOBz的代谢物。化学相关的甲氧基化四溴到六溴-DiPhOBz是鲱鸥中的已知污染物。总体上,目前对于这些多溴化(PB)DiPhOBz基化合物的生物效应一无所知。本实验研究的2,2-电位thyroidogenicity',2“,4四溴(TB)与它的沿-DiPhOBz对-甲氧基(MEO) -和羟基- (OH)-analogues,使用体外人甲状腺激素(TH)转运蛋白运甲状腺素蛋白(hTTR)和白蛋白(hALB)的竞争性蛋白结合测定。发现该模型对-OH-TB-DiPhOBz能够与甲状腺素(T4)竞争hTTR和hALB上的结合位点。在计算机上还使用3D同源模型对海鸥TTR进行了分析,以预测这些基于TB-DiPhOBz的化合物是否也可以作为禽类TH转运蛋白的配体,尽管与hTTR在进化上有所不同。该分析发现,所有三种TB-DiPhOBz类似物都是鸥TTR的潜在配体,并且与TH的结合效率相似。结果表明这些配体的结合亲和力与结构有关,并且表明这些污染物可能与哺乳动物和禽类甲状腺功能相互作用。
更新日期:2018-01-16
中文翻译:

溴化聚苯醚污染物与人和鸥甲状腺激素转运蛋白的体外和计算机竞争性结合
Tetradecabromo-1,4-diphenoxybenzene(TeDB-DiPhOBz)是一种高度溴化的添加剂阻燃剂(FR)。在基于鲱鸥(Larus argentatus)的肝微粒体测定法中,将TeDB-DiPhOBz的脱溴光降解物在体外进行羟基化,包括一种被鉴定为4” -OH-2,2',2”,4-四溴-DiPhOBz的代谢物。化学相关的甲氧基化四溴到六溴-DiPhOBz是鲱鸥中的已知污染物。总体上,目前对于这些多溴化(PB)DiPhOBz基化合物的生物效应一无所知。本实验研究的2,2-电位thyroidogenicity',2“,4四溴(TB)与它的沿-DiPhOBz对-甲氧基(MEO) -和羟基- (OH)-analogues,使用体外人甲状腺激素(TH)转运蛋白运甲状腺素蛋白(hTTR)和白蛋白(hALB)的竞争性蛋白结合测定。发现该模型对-OH-TB-DiPhOBz能够与甲状腺素(T4)竞争hTTR和hALB上的结合位点。在计算机上还使用3D同源模型对海鸥TTR进行了分析,以预测这些基于TB-DiPhOBz的化合物是否也可以作为禽类TH转运蛋白的配体,尽管与hTTR在进化上有所不同。该分析发现,所有三种TB-DiPhOBz类似物都是鸥TTR的潜在配体,并且与TH的结合效率相似。结果表明这些配体的结合亲和力与结构有关,并且表明这些污染物可能与哺乳动物和禽类甲状腺功能相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号