当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism of N2 Reduction Catalyzed by Fe-Nitrogenase Involves Reductive Elimination of H2
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.biochem.7b01142 Derek F. Harris 1 , Dmitriy A. Lukoyanov 2 , Sudipta Shaw 1 , Phil Compton 2 , Monika Tokmina-Lukaszewska 3 , Brian Bothner 3 , Neil Kelleher 2 , Dennis R. Dean 4 , Brian M. Hoffman 2 , Lance C. Seefeldt 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.biochem.7b01142 Derek F. Harris 1 , Dmitriy A. Lukoyanov 2 , Sudipta Shaw 1 , Phil Compton 2 , Monika Tokmina-Lukaszewska 3 , Brian Bothner 3 , Neil Kelleher 2 , Dennis R. Dean 4 , Brian M. Hoffman 2 , Lance C. Seefeldt 1
Affiliation
|
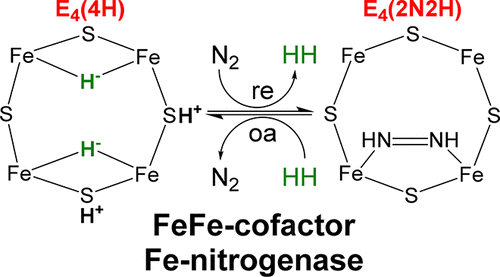
Of the three forms of nitrogenase (Mo-nitrogenase, V-nitrogenase, and Fe-nitrogenase), Fe-nitrogenase has the poorest ratio of N2 reduction relative to H2 evolution. Recent work on the Mo-nitrogenase has revealed that reductive elimination of two bridging Fe–H–Fe hydrides on the active site FeMo-cofactor to yield H2 is a key feature in the N2 reduction mechanism. The N2 reduction mechanism for the Fe-nitrogenase active site FeFe-cofactor was unknown. Here, we have purified both component proteins of the Fe-nitrogenase system, the electron-delivery Fe protein (AnfH) plus the catalytic FeFe protein (AnfDGK), and established its mechanism of N2 reduction. Inductively coupled plasma optical emission spectroscopy and mass spectrometry show that the FeFe protein component does not contain significant amounts of Mo or V, thus ruling out a requirement of these metals for N2 reduction. The fully functioning Fe-nitrogenase system was found to have specific activities for N2 reduction (1 atm) of 181 ± 5 nmol NH3 min–1 mg–1 FeFe protein, for proton reduction (in the absence of N2) of 1085 ± 41 nmol H2 min–1 mg–1 FeFe protein, and for acetylene reduction (0.3 atm) of 306 ± 3 nmol C2H4 min–1 mg–1 FeFe protein. Under turnover conditions, N2 reduction is inhibited by H2 and the enzyme catalyzes the formation of HD when presented with N2 and D2. These observations are explained by the accumulation of four reducing equivalents as two metal-bound hydrides and two protons at the FeFe-cofactor, with activation for N2 reduction occurring by reductive elimination of H2.
中文翻译:

Fe-固氮酶催化N 2还原的机理涉及H 2的还原消除
在三种形式的固氮酶(Mo-固氮酶,V-固氮酶和Fe-固氮酶)中,Fe-固氮酶相对于H 2析出的N 2还原率最差。Mo固氮酶的最新研究表明,在活性位点FeMo辅因子上消除两个桥接的Fe–H–Fe氢化物以产生H 2是N 2还原机制的关键特征。Fe固氮酶活性位点FeFe辅因子的N 2还原机理尚不清楚。在这里,我们纯化了铁氮酶系统的两个组成蛋白,电子传递铁蛋白(AnfH)和催化铁蛋白FeFe(AnfDGK),并确定了其N 2的作用机理。减少。电感耦合等离子体发射光谱法和质谱法表明,FeFe蛋白成分不包含大量的Mo或V,因此排除了这些金属对N 2还原的需求。已发现功能齐全的Fe固氮酶系统具有181±5 nmol NH 3 min –1 mg –1 FeFe蛋白的N 2还原(1 atm)的特定活性,对于1085的质子还原(无N 2)具有特定的活性。±41 nmol H 2 min –1 mg –1 FeFe蛋白,乙炔还原(0.3 atm)为306±3 nmol C 2 H 4 min –1mg –1 FeFe蛋白。在周转条件下,N 2的还原被H 2抑制,当与N 2和D 2一起存在时,该酶催化HD的形成。这些观察结果通过FeFe-辅因子在FeFe-辅因子上作为两个金属结合的氢化物和两个质子的四个还原当量的积累而得以解释,其中N 2还原的活化通过还原性消除H 2发生。
更新日期:2018-01-17
中文翻译:

Fe-固氮酶催化N 2还原的机理涉及H 2的还原消除
在三种形式的固氮酶(Mo-固氮酶,V-固氮酶和Fe-固氮酶)中,Fe-固氮酶相对于H 2析出的N 2还原率最差。Mo固氮酶的最新研究表明,在活性位点FeMo辅因子上消除两个桥接的Fe–H–Fe氢化物以产生H 2是N 2还原机制的关键特征。Fe固氮酶活性位点FeFe辅因子的N 2还原机理尚不清楚。在这里,我们纯化了铁氮酶系统的两个组成蛋白,电子传递铁蛋白(AnfH)和催化铁蛋白FeFe(AnfDGK),并确定了其N 2的作用机理。减少。电感耦合等离子体发射光谱法和质谱法表明,FeFe蛋白成分不包含大量的Mo或V,因此排除了这些金属对N 2还原的需求。已发现功能齐全的Fe固氮酶系统具有181±5 nmol NH 3 min –1 mg –1 FeFe蛋白的N 2还原(1 atm)的特定活性,对于1085的质子还原(无N 2)具有特定的活性。±41 nmol H 2 min –1 mg –1 FeFe蛋白,乙炔还原(0.3 atm)为306±3 nmol C 2 H 4 min –1mg –1 FeFe蛋白。在周转条件下,N 2的还原被H 2抑制,当与N 2和D 2一起存在时,该酶催化HD的形成。这些观察结果通过FeFe-辅因子在FeFe-辅因子上作为两个金属结合的氢化物和两个质子的四个还原当量的积累而得以解释,其中N 2还原的活化通过还原性消除H 2发生。


























 京公网安备 11010802027423号
京公网安备 11010802027423号