当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Identification and Kinetic Analysis of the in Vitro Products Formed by Reaction of Bisphenol A-3,4-quinone with N-Acetylcysteine and Glutathione
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.chemrestox.7b00239 Douglas E. Stack 1 , John A. Conrad 1 , Bejan Mahmud 1
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.chemrestox.7b00239 Douglas E. Stack 1 , John A. Conrad 1 , Bejan Mahmud 1
Affiliation
|
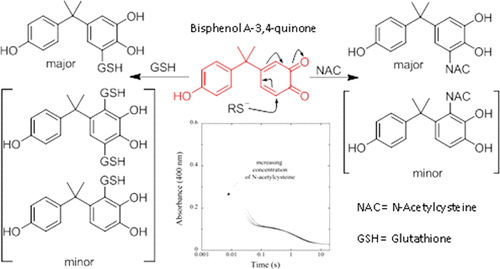
Bisphenol A (BPA) has received considerable attention as an endocrine disrupting chemical and a possible substrate for genotoxic metabolites. BPA metabolism leads to formation of electrophilic o-quinones cable of binding to DNA and other endogenous nucleophiles. We have structurally identified the products resulting from the reaction of bisphenol A-3,4-quinone (BPAQ) with N-acetylcysteine (NAC) and glutathione (GSH). The major and minor isomers are both the result of 1,6-conjugate addition and are produced almost instantly in high yield. Reactions using 1.3 equiv of GSH showed the presence of a bis-glutathionyl adduct which was not observed using higher GSH concentration relative to BPAQ. NAC reactions with BPAQ showed no bis-N-acetylcysteinyl adducts. Stopped-flow kinetic analysis reveals the 1,6-conjugate additions to be reversible with a forward free energy of activation of 9.2 and 7.8 kcal/mol for the NAC and GSH reactions, respectively. The bimolecular forward rate constant at 19.4 °C was approximately three time faster for GSH compared to NAC, 1547 vs 496 M–1 s–1. The free energy of activation for the reverse reactions were similar, 11.7 and 11.2 kcal/mol for NAC and GSH, respectively. We plan to use this model system to further explore the mechanism of adduct formation between sulfur nucleophiles and o-quinones and the resulting chemical properties of both NAC and GSH adducts.
中文翻译:

双酚A-3,4-醌与N-乙酰半胱氨酸和谷胱甘肽反应形成的体外产物的结构鉴定和动力学分析
双酚A(BPA)作为破坏内分泌的化学物质和可能的遗传毒性代谢物而受到了广泛关注。BPA代谢导致形成电的ö -quinones结合DNA和其它内源性亲核试剂电缆。我们已经从结构上确定了双酚A-3,4-醌(BPAQ)与N-乙酰半胱氨酸(NAC)和谷胱甘肽(GSH)反应生成的产物。主要和次要异构体都是1,6-共轭物加成的结果,几乎可以立即以高收率生产。使用1.3当量的GSH的反应表明存在双谷胱甘肽加合物,相对于BPAQ,使用较高的GSH浓度未观察到。NAC与BPAQ的反应显示没有bis- N-乙酰半胱氨酸加合物。停止流动动力学分析表明,对于NAC和GSH反应,添加的1,6-共轭物是可逆的,激活的正向自由能分别为9.2和7.8 kcal / mol。与NAC相比,GSH的双分子正向速率常数恒定在19.4°C约快三倍,分别为1547和496 M –1 s –1。逆反应的活化自由能相近,NAC和GSH分别为11.7 kcal / mol和11.2 kcal / mol。我们计划使用该模型系统来进一步探索硫亲核体与邻醌之间加合物形成的机理,以及NAC和GSH加合物的化学性质。
更新日期:2018-01-16
中文翻译:

双酚A-3,4-醌与N-乙酰半胱氨酸和谷胱甘肽反应形成的体外产物的结构鉴定和动力学分析
双酚A(BPA)作为破坏内分泌的化学物质和可能的遗传毒性代谢物而受到了广泛关注。BPA代谢导致形成电的ö -quinones结合DNA和其它内源性亲核试剂电缆。我们已经从结构上确定了双酚A-3,4-醌(BPAQ)与N-乙酰半胱氨酸(NAC)和谷胱甘肽(GSH)反应生成的产物。主要和次要异构体都是1,6-共轭物加成的结果,几乎可以立即以高收率生产。使用1.3当量的GSH的反应表明存在双谷胱甘肽加合物,相对于BPAQ,使用较高的GSH浓度未观察到。NAC与BPAQ的反应显示没有bis- N-乙酰半胱氨酸加合物。停止流动动力学分析表明,对于NAC和GSH反应,添加的1,6-共轭物是可逆的,激活的正向自由能分别为9.2和7.8 kcal / mol。与NAC相比,GSH的双分子正向速率常数恒定在19.4°C约快三倍,分别为1547和496 M –1 s –1。逆反应的活化自由能相近,NAC和GSH分别为11.7 kcal / mol和11.2 kcal / mol。我们计划使用该模型系统来进一步探索硫亲核体与邻醌之间加合物形成的机理,以及NAC和GSH加合物的化学性质。



























 京公网安备 11010802027423号
京公网安备 11010802027423号