Applied Catalysis A: General ( IF 5.5 ) Pub Date : 2017-12-27 , DOI: 10.1016/j.apcata.2017.12.016 Bohan Jia , Jiaxiu Guo , Hongdi Luo , Song Shu , Ningjie Fang , Jianjun Li
|
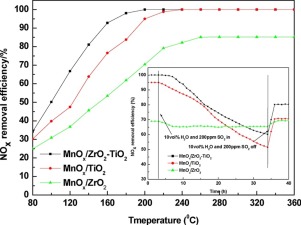
MnOx/TiO2, MnOx/ZrO2 and MnOx/ZrO2–TiO2 were prepared by impregnation and NO removal was evaluated in a fixed‒bed reactor under simulated reactive gas. All samples were characterized by various technologies. The results showed that MnOx/ZrO2–TiO2 had good selective catalytic reduction activity between 80–360 °C and excellent resistance to H2O at 200 °C for 30 h and achieved 80% NOx removal efficiency at 140 °C. The NO removal efficiency of MnOx/ZrO2 was only 45–80%, and that of MnOx/TiO2 was 100% at 240–360 °C. However, their tolerance to H2O was not as good as MnOx/ZrO2–TiO2. When SO2 was induced into reactive gas, the NO removal efficiency of MnOx/ZrO2 at 200 °C was maintained 70% for 34 h, but that of MnOx/TiO2 decreased rapidly. MnOx/ZrO2–TiO2 maintained 100% NO removal efficiency for 5 h and decreased gradually. After SO2 was stopped, NO removal ability wasn’t recovered, indicating that deactivation of catalyst was irreversible. TiO2 can improve the texture properties, and the surface area and total pore volume of MnOx/TiO2 reached 313 m2/g and 0.38 m3/g. The existence of zirconium oxide significantly increased the concentration of Mn4+ and Oβ on the surface of catalysts. MnOx/ZrO2–TiO2 had wide surface acidity, which improved NH3 adsorption. After SO2 resistance testing, Mn4+ content on the surface of catalysts decreased whereas Mn3+ obviously increased. SO2 could be oxidized by MnOx to SO3, and SO3 was adsorbed on ZrO2 or TiO2 to form zirconium sulfate or titanyl sulfate. (NH4)2SO4 was formed on singe ZrO2 or TiO2 carrier. These sulfates increased the surface acidity, resulting in a change of NO removal ability.
中文翻译:

MnO x / TiO 2,MnO x / ZrO 2和MnO x / ZrO 2 –TiO 2的NO去除及对SO 2和H 2 O的抵抗性的研究
通过浸渍制备MnO x / TiO 2,MnO x / ZrO 2和MnO x / ZrO 2 -TiO 2,并在模拟反应气体下在固定床反应器中评估NO的去除率。所有样品均采用各种技术进行了表征。结果表明,MnO x / ZrO 2 -TiO 2在80-360°C之间具有良好的选择性催化还原活性,在200°C下30 h具有出色的抗H 2 O性能,在140°C下达到80%的NO x去除率。MnO x / ZrO 2的NO去除效率在240-360°C时,MnO x / TiO 2的含量仅为45-80%,而100%为MnO x / TiO 2。但是,它们对H 2 O的耐受性不如MnO x / ZrO 2 -TiO 2。当SO 2被引入反应气体中时,MnO x / ZrO 2在200°C下的NO去除效率保持70%达34 h,而MnO x / TiO 2的NO去除效率却迅速下降。MnO x / ZrO 2 -TiO 2维持100%的NO去除效率达5 h,并逐渐降低。SO 2之后停止,没有去除能力恢复,表明催化剂的失活是不可逆的。TiO 2可以改善织构性质,并且MnO x / TiO 2的表面积和总孔体积分别达到313m 2 / g和0.38m 3 / g。氧化锆的存在显著增加Mn的浓度4+和O β催化剂的表面上。MnO x / ZrO 2 -TiO 2具有较宽的表面酸性,从而改善了NH 3的吸附。经过SO 2电阻测试后,Mn 4+催化剂表面的含量下降,而Mn 3+明显增加。SO 2可以被MnO x氧化为SO 3,SO 3吸附在ZrO 2或TiO 2上形成硫酸锆或硫酸氧钛。在单一的ZrO 2或TiO 2载体上形成了(NH 4)2 SO 4。这些硫酸盐增加了表面酸度,导致NO去除能力的改变。



























 京公网安备 11010802027423号
京公网安备 11010802027423号