当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Clean Electrocatalysis in a Li2O2 Redox-Based Li–O2 Battery Built with a Hydrate-Melt Electrolyte
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acscatal.7b02960 Shichao Wu 1 , Yu Qiao 1 , Sixie Yang 2 , Jing Tang 3 , Ping He 2 , Haoshen Zhou 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acscatal.7b02960 Shichao Wu 1 , Yu Qiao 1 , Sixie Yang 2 , Jing Tang 3 , Ping He 2 , Haoshen Zhou 1, 2
Affiliation
|
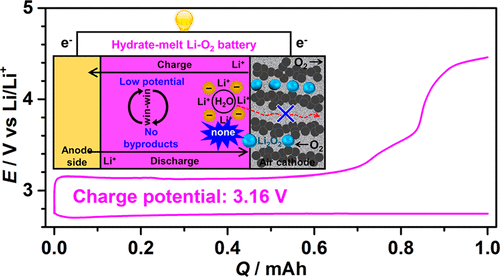
The electrocatalysis of the oxygen reduction reaction and oxygen evolution reaction in nonaqueous Li–O2 batteries suffers from severe side reactions and high charge potential. Herein, we design an unreported hydrate-melt Li–O2 battery without use of an unstable organic solvent. Its redox reaction depends on the formation of Li2O2 in high yield (96%) and its full decomposition. The designed battery shows an ultralow charge potential of ∼3.16 V, a high discharge capacity of 38 mAh cm–2, and stable cycling ability. After careful comparison of the discharge and charge products by XRD, SEM, Raman, FTIR, NMR, and quantitative titration, the great improvement in performance is attributed to the efficient avoidance of side reactions related to organic solvent degradation, which may be the primary cause of the high charge potential in a nonaqueous Li–O2 battery. These results should initiate a deep understanding of Li–O2 batteries and indicate another strategy toward practical Li–air batteries with moisture-proof properties and high safety.
中文翻译:

内置水合电解质的Li 2 O 2氧化还原基Li–O 2电池中的清洁电催化
非水Li–O 2电池中氧还原反应和氧释放反应的电催化存在严重的副反应和高充电电位。在此,我们设计了一种未报告的水合熔融Li–O 2电池,而不使用不稳定的有机溶剂。其氧化还原反应取决于高产率(96%)Li 2 O 2的形成及其完全分解。设计的电池显示约3.16 V的超低充电电位,38 mAh cm –2的高放电容量,循环能力稳定。通过XRD,SEM,拉曼光谱,FTIR,NMR和定量滴定仔细比较放电和电荷产物后,性能的极大提高归因于有效避免了与有机溶剂降解有关的副反应,这可能是主要原因非水Li–O 2电池中的高充电电位。这些结果将使人们对Li-O 2电池有更深入的了解,并为朝着具有防潮性能和高安全性的实用锂空气电池发展提供另一种策略。
更新日期:2018-01-11
中文翻译:

内置水合电解质的Li 2 O 2氧化还原基Li–O 2电池中的清洁电催化
非水Li–O 2电池中氧还原反应和氧释放反应的电催化存在严重的副反应和高充电电位。在此,我们设计了一种未报告的水合熔融Li–O 2电池,而不使用不稳定的有机溶剂。其氧化还原反应取决于高产率(96%)Li 2 O 2的形成及其完全分解。设计的电池显示约3.16 V的超低充电电位,38 mAh cm –2的高放电容量,循环能力稳定。通过XRD,SEM,拉曼光谱,FTIR,NMR和定量滴定仔细比较放电和电荷产物后,性能的极大提高归因于有效避免了与有机溶剂降解有关的副反应,这可能是主要原因非水Li–O 2电池中的高充电电位。这些结果将使人们对Li-O 2电池有更深入的了解,并为朝着具有防潮性能和高安全性的实用锂空气电池发展提供另一种策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号