当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unraveling the Critical Role Played by Ado762′OH in the Post-Transfer Editing by Archaeal Threonyl-tRNA Synthetase
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acs.jpcb.7b10254 Mohamed M. Aboelnga 1, 2 , John J. Hayward 1 , James W. Gauld 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acs.jpcb.7b10254 Mohamed M. Aboelnga 1, 2 , John J. Hayward 1 , James W. Gauld 1
Affiliation
|
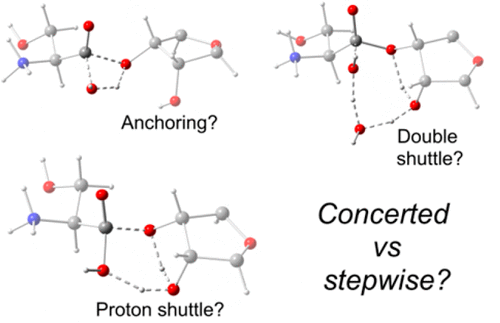
Archaeal threonyl-tRNA synthetase (ThrRS) possesses an editing active site wherein tRNAThr that has been misaminoacylated with serine (i.e., Ser-tRNAThr) is hydrolytically cleaved to serine and tRNAThr. It has been suggested that the free ribose sugar hydroxyl of Ado76 of the tRNAThr (Ado762′OH) is the mechanistic base, promoting hydrolysis by orienting a nucleophilic water near the scissile Ser-tRNAThr ester bond. We have performed a computational study, involving molecular dynamics (MD) and hybrid ONIOM quantum mechanics/molecular mechanics (QM/MM) methods, considering all possible editing mechanisms to gain an understanding of the role played by Ado762′OH group. More specifically, a range of concerted or stepwise mechanisms involving four-, six-, or eight-membered transition structures (total of seven mechanisms) were considered. In addition, these seven mechanisms were fully optimized using three different DFT functionals, namely, B3LYP, M06-2X, and M06-HF. The M06-HF functional gave the most feasible energy barriers followed by the M06-2X functional. The most favorable mechanism proceeds stepwise through two six-membered ring transition states in which the Ado762′OH group participates, overall, as a shuttle for the proton transfer from the nucleophilic H2O to the bridging oxygen (Ado763′O) of the substrate. More specifically, in the first step, which has a barrier of 25.9 kcal/mol, the Ado762′-OH group accepts a proton from the attacking nucleophilic water while concomitantly transferring its proton onto the substrates C–Ocarb center. Then, in the second step, which also proceeds with a barrier of 25.9 kcal/mol, the Ado762′-OH group transfers its proton on the adjacent Ado763′-oxygen, cleaving the scissile Ccarb–O3′Ado76 bond, while concomitantly accepting a proton from the previously formed C–OcarbH group.
中文翻译:

阐明Ado76 2'OH在古细菌苏氨酸-tRNA合成酶的转移后编辑中所起的关键作用
古细菌苏氨酰tRNA合成酶酶(ThrRS)具有一个编辑的活性位点,其中的tRNA苏氨酸已被misaminoacylated用丝氨酸(即,丝氨酸酰-tRNA苏氨酸)被水解裂解以丝氨酸和tRNA苏氨酸。已经提出,tRNA Thr的Ado76的游离核糖糖羟基(Ado76 2'OH)是机制碱基,通过使易裂的Ser-tRNA Thr酯键附近的亲核水取向来促进水解。我们进行了一项计算研究,涉及分子动力学(MD)和混合ONIOM量子力学/分子力学(QM / MM)方法,并考虑了所有可能的编辑机制以了解Ado76的作用2′OH基团。更具体地说,考虑了涉及四元,六元或八元过渡结构(总共七个机制)的一系列协调或逐步机制。此外,使用三种不同的DFT功能(即B3LYP,M06-2X和M06-HF)对这七个机制进行了完全优化。M06-HF功能提供了最可行的能垒,其次是M06-2X功能。最有利的机制逐步进行,通过两个六元环跃迁状态,其中Ado76 2'OH基团总体上作为质子从亲核H 2 O转移至桥联氧(Ado76 3'O)的穿梭体而参与。基板。更具体地,在具有25.9 kcal / mol的势垒的第一步中,Ado76 2'-OH基团从侵蚀性亲核水中接受质子,同时将其质子转移到基质C–O碳中心上。然后,在第二步骤中,这也与25.9阻挡前进千卡/摩尔,所述Ado76 2'-OH基团转移其上的相邻质子Ado76 3'-氧,切割易切断Ç碳水化合物-O3' Ado76键,而同时接受先前形成的C–O carb H基团的质子。
更新日期:2018-01-11
中文翻译:

阐明Ado76 2'OH在古细菌苏氨酸-tRNA合成酶的转移后编辑中所起的关键作用
古细菌苏氨酰tRNA合成酶酶(ThrRS)具有一个编辑的活性位点,其中的tRNA苏氨酸已被misaminoacylated用丝氨酸(即,丝氨酸酰-tRNA苏氨酸)被水解裂解以丝氨酸和tRNA苏氨酸。已经提出,tRNA Thr的Ado76的游离核糖糖羟基(Ado76 2'OH)是机制碱基,通过使易裂的Ser-tRNA Thr酯键附近的亲核水取向来促进水解。我们进行了一项计算研究,涉及分子动力学(MD)和混合ONIOM量子力学/分子力学(QM / MM)方法,并考虑了所有可能的编辑机制以了解Ado76的作用2′OH基团。更具体地说,考虑了涉及四元,六元或八元过渡结构(总共七个机制)的一系列协调或逐步机制。此外,使用三种不同的DFT功能(即B3LYP,M06-2X和M06-HF)对这七个机制进行了完全优化。M06-HF功能提供了最可行的能垒,其次是M06-2X功能。最有利的机制逐步进行,通过两个六元环跃迁状态,其中Ado76 2'OH基团总体上作为质子从亲核H 2 O转移至桥联氧(Ado76 3'O)的穿梭体而参与。基板。更具体地,在具有25.9 kcal / mol的势垒的第一步中,Ado76 2'-OH基团从侵蚀性亲核水中接受质子,同时将其质子转移到基质C–O碳中心上。然后,在第二步骤中,这也与25.9阻挡前进千卡/摩尔,所述Ado76 2'-OH基团转移其上的相邻质子Ado76 3'-氧,切割易切断Ç碳水化合物-O3' Ado76键,而同时接受先前形成的C–O carb H基团的质子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号