当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Potassium Amide‐Catalyzed Benzylic C−H Bond Addition of Alkylpyridines to Styrenes
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-01-16 , DOI: 10.1002/anie.201710128 Dan-Dan Zhai 1 , Xiang-Yu Zhang 1 , Yu-Feng Liu 1 , Lei Zheng 1 , Bing-Tao Guan 1, 2
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-01-16 , DOI: 10.1002/anie.201710128 Dan-Dan Zhai 1 , Xiang-Yu Zhang 1 , Yu-Feng Liu 1 , Lei Zheng 1 , Bing-Tao Guan 1, 2
Affiliation
|
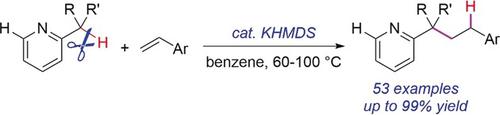
The benzylic functionalization of alkylpyridines is an important pathway for pyridine derivatives synthesis. The reaction partners, however, were mostly limited to highly reactive polar electrophiles. Herein, we report a potassium amide‐catalyzed selective benzylic C−H bond addition of alkylpyridines to styrenes. Potassium bis(trimethylsilyl)amide (KHMDS), a readily available Brønsted base, showed excellent catalytic activity and chemoselectivity. A series of alkylpyridine derivatives, including benzylic quaternary carbon substituted pyridines, were obtained in good to high yield. Preliminary mechanistic studies revealed that the deprotonation equilibrium is probably responsible for the excellent selectivity.
中文翻译:

酰胺钾催化的烷基吡啶对苯乙烯的苯甲酰CH键加氢
烷基吡啶的苄基官能化是吡啶衍生物合成的重要途径。然而,反应伙伴主要限于高反应性的极性亲电试剂。在本文中,我们报道了烷基吡啶向苯乙烯中的钾酰胺催化选择性苄基CH键加成。双(三甲基甲硅烷基)酰胺钾(KHMDS)是一种容易获得的布朗斯台德碱,具有出色的催化活性和化学选择性。以良好或高收率获得了一系列烷基吡啶衍生物,包括苄基季碳取代的吡啶。初步的机理研究表明,去质子平衡可能是造成极好的选择性的原因。
更新日期:2018-01-16
中文翻译:

酰胺钾催化的烷基吡啶对苯乙烯的苯甲酰CH键加氢
烷基吡啶的苄基官能化是吡啶衍生物合成的重要途径。然而,反应伙伴主要限于高反应性的极性亲电试剂。在本文中,我们报道了烷基吡啶向苯乙烯中的钾酰胺催化选择性苄基CH键加成。双(三甲基甲硅烷基)酰胺钾(KHMDS)是一种容易获得的布朗斯台德碱,具有出色的催化活性和化学选择性。以良好或高收率获得了一系列烷基吡啶衍生物,包括苄基季碳取代的吡啶。初步的机理研究表明,去质子平衡可能是造成极好的选择性的原因。



























 京公网安备 11010802027423号
京公网安备 11010802027423号