当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric [4+2] Cycloaddition of in Situ Generated o-Quinone Methide Imines with o-Hydroxystyrenes: Diastereo- and Enantioselective Construction of Tetrahydroquinoline Frameworks
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.joc.7b02533 Lin-Zhi Li 1 , Cong-Shuai Wang 1 , Wei-Feng Guo 1 , Guang-Jian Mei 1 , Feng Shi 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.joc.7b02533 Lin-Zhi Li 1 , Cong-Shuai Wang 1 , Wei-Feng Guo 1 , Guang-Jian Mei 1 , Feng Shi 1
Affiliation
|
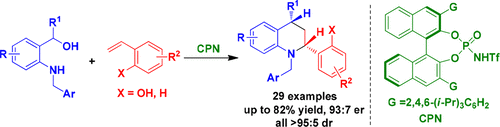
A catalytic asymmetric [4+2] cycloaddition of ortho-quinone methide imines in situ generated from o-aminobenzyl alcohols with o-hydroxystyrenes has been established under the catalysis of chiral phosphoramide, which afforded chiral tetrahydroquinolines in moderate to good yields, good enantioselectivities, and excellent diastereoselectivities (up to 82% yield, 93:7 er, all >95:5 dr). In this catalytic asymmetric [4+2] cycloaddition, the hydrogen-bonding interaction between chiral phosphoramide and two substrates was proposed to play a crucial role in controlling the enantioselectivity. This reaction not only provides a useful approach for constructing chiral tetrahydroquinoline frameworks, but also demonstrates the great practicability of ortho-quinone methide imines in catalytic asymmetric cycloadditions.
中文翻译:

原位生成的邻苯二甲酰亚胺亚胺与邻羟基苯乙烯的催化不对称[4 + 2]环加成反应:四氢喹啉骨架的非对映和对映选择性结构
在手性磷酰胺的催化下,建立了由邻氨基苄醇与邻羟基苯乙烯催化原位生成的邻醌甲基亚胺催化不对称[4 + 2]环加成反应,该方法可提供中等至良好收率的手性四氢喹啉,良好的对映选择性,和出色的非对映选择性(产率高达82%,93:7 er,所有> 95:5 dr)。在这种催化不对称的[4 + 2]环加成反应中,手性磷酰胺与两种底物之间的氢键相互作用被认为在控制对映选择性中起着至关重要的作用。该反应不仅为构建手性四氢喹啉骨架提供了一种有用的方法,而且证明了邻位的巨大实用性。-醌甲基亚胺在催化不对称环加成反应中的作用。
更新日期:2018-01-10
中文翻译:

原位生成的邻苯二甲酰亚胺亚胺与邻羟基苯乙烯的催化不对称[4 + 2]环加成反应:四氢喹啉骨架的非对映和对映选择性结构
在手性磷酰胺的催化下,建立了由邻氨基苄醇与邻羟基苯乙烯催化原位生成的邻醌甲基亚胺催化不对称[4 + 2]环加成反应,该方法可提供中等至良好收率的手性四氢喹啉,良好的对映选择性,和出色的非对映选择性(产率高达82%,93:7 er,所有> 95:5 dr)。在这种催化不对称的[4 + 2]环加成反应中,手性磷酰胺与两种底物之间的氢键相互作用被认为在控制对映选择性中起着至关重要的作用。该反应不仅为构建手性四氢喹啉骨架提供了一种有用的方法,而且证明了邻位的巨大实用性。-醌甲基亚胺在催化不对称环加成反应中的作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号