当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deciphering the Dynamic Interaction Profile of an Intrinsically Disordered Protein by NMR Exchange Spectroscopy
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-11 , DOI: 10.1021/jacs.7b12407 Elise Delaforge 1 , Jaka Kragelj 1 , Laura Tengo 1 , Andrés Palencia 2 , Sigrid Milles 1 , Guillaume Bouvignies 3, 4 , Nicola Salvi 1 , Martin Blackledge 1 , Malene Ringkjøbing Jensen 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-11 , DOI: 10.1021/jacs.7b12407 Elise Delaforge 1 , Jaka Kragelj 1 , Laura Tengo 1 , Andrés Palencia 2 , Sigrid Milles 1 , Guillaume Bouvignies 3, 4 , Nicola Salvi 1 , Martin Blackledge 1 , Malene Ringkjøbing Jensen 1
Affiliation
|
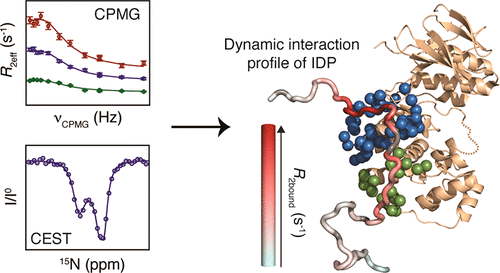
Intrinsically disordered proteins (IDPs) display a large number of interaction modes including folding-upon-binding, binding without major structural transitions, or binding through highly dynamic, so-called fuzzy, complexes. The vast majority of experimental information about IDP binding modes have been inferred from crystal structures of proteins in complex with short peptides of IDPs. However, crystal structures provide a mainly static view of the complexes and do not give information about the conformational dynamics experienced by the IDP in the bound state. Knowledge of the dynamics of IDP complexes is of fundamental importance to understand how IDPs engage in highly specific interactions without concomitantly high binding affinity. Here, we combine rotating-frame R1ρ, Carr-Purcell-Meiboom Gill relaxation dispersion as well as chemical exchange saturation transfer to decipher the dynamic interaction profile of an IDP in complex with its partner. We apply the approach to the dynamic signaling complex formed between the mitogen-activated protein kinase (MAPK) p38α and the intrinsically disordered regulatory domain of the MAPK kinase MKK4. Our study demonstrates that MKK4 employs a subtle combination of interaction modes in order to bind to p38α, leading to a complex displaying significantly different dynamics across the bound regions.
中文翻译:

通过 NMR 交换光谱法破译固有无序蛋白质的动态相互作用谱
固有无序蛋白 (IDP) 显示出大量的相互作用模式,包括结合后折叠、没有主要结构转变的结合或通过高度动态的、所谓的模糊复合物结合。绝大多数关于 IDP 结合模式的实验信息都是从与 IDP 短肽复合的蛋白质的晶体结构中推断出来的。然而,晶体结构提供了复合物的主要静态视图,并没有提供有关 IDP 在结合状态下所经历的构象动力学的信息。了解 IDP 复合物的动力学对于了解 IDP 如何在不伴随高结合亲和力的情况下参与高度特异性的相互作用具有根本重要性。在这里,我们结合旋转坐标系 R1ρ,Carr-Purcell-Meiboom Gill 弛豫分散以及化学交换饱和转移,以破译 IDP 与其伴侣复合体的动态相互作用曲线。我们将该方法应用于在丝裂原活化蛋白激酶 (MAPK) p38α 和 MAPK 激酶 MKK4 的内在无序调节域之间形成的动态信号复合物。我们的研究表明,MKK4 采用微妙的相互作用模式组合来与 p38α 结合,导致复杂的结合区域显示出显着不同的动态。我们将该方法应用于在丝裂原活化蛋白激酶 (MAPK) p38α 和 MAPK 激酶 MKK4 的内在无序调节域之间形成的动态信号复合物。我们的研究表明,MKK4 采用微妙的相互作用模式组合来与 p38α 结合,导致复杂的结合区域显示出显着不同的动态。我们将该方法应用于在丝裂原活化蛋白激酶 (MAPK) p38α 和 MAPK 激酶 MKK4 的内在无序调节域之间形成的动态信号复合物。我们的研究表明,MKK4 采用微妙的相互作用模式组合来与 p38α 结合,导致复杂的结合区域显示出显着不同的动态。
更新日期:2018-01-11
中文翻译:

通过 NMR 交换光谱法破译固有无序蛋白质的动态相互作用谱
固有无序蛋白 (IDP) 显示出大量的相互作用模式,包括结合后折叠、没有主要结构转变的结合或通过高度动态的、所谓的模糊复合物结合。绝大多数关于 IDP 结合模式的实验信息都是从与 IDP 短肽复合的蛋白质的晶体结构中推断出来的。然而,晶体结构提供了复合物的主要静态视图,并没有提供有关 IDP 在结合状态下所经历的构象动力学的信息。了解 IDP 复合物的动力学对于了解 IDP 如何在不伴随高结合亲和力的情况下参与高度特异性的相互作用具有根本重要性。在这里,我们结合旋转坐标系 R1ρ,Carr-Purcell-Meiboom Gill 弛豫分散以及化学交换饱和转移,以破译 IDP 与其伴侣复合体的动态相互作用曲线。我们将该方法应用于在丝裂原活化蛋白激酶 (MAPK) p38α 和 MAPK 激酶 MKK4 的内在无序调节域之间形成的动态信号复合物。我们的研究表明,MKK4 采用微妙的相互作用模式组合来与 p38α 结合,导致复杂的结合区域显示出显着不同的动态。我们将该方法应用于在丝裂原活化蛋白激酶 (MAPK) p38α 和 MAPK 激酶 MKK4 的内在无序调节域之间形成的动态信号复合物。我们的研究表明,MKK4 采用微妙的相互作用模式组合来与 p38α 结合,导致复杂的结合区域显示出显着不同的动态。我们将该方法应用于在丝裂原活化蛋白激酶 (MAPK) p38α 和 MAPK 激酶 MKK4 的内在无序调节域之间形成的动态信号复合物。我们的研究表明,MKK4 采用微妙的相互作用模式组合来与 p38α 结合,导致复杂的结合区域显示出显着不同的动态。



























 京公网安备 11010802027423号
京公网安备 11010802027423号