Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2017-12-23 , DOI: 10.1016/j.bmcl.2017.12.048 Sze Wei Leong , Faridah Abas , Kok Wai Lam , Khatijah Yusoff
|
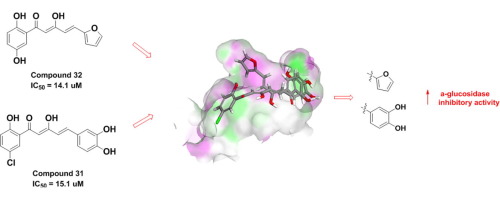
A series of thirty-four diarylpentanoids derivatives were synthesized and evaluated for their α-glucosidase inhibitory activity. Eleven compounds (19, 20, 21, 24, 27, 28, 29, 31, 32, 33 and 34) were found to significantly inhibit α-glucosidase in which compounds 28, 31 and 32 demonstrated the highest activity with IC50 values ranging from 14.1 to 15.1 µM. Structure-activity comparison shows that multiple hydroxy groups are essential for α-glucosidase inhibitory activity. Meanwhile, 3,4-dihydroxyphenyl and furanyl moieties were found to be crucial in improving α-glucosidase inhibition. Molecular docking analyses further confirmed the critical role of both 3,4-dihydroxyphenyl and furanyl moieties as they bound to α-glucosidase active site in different mode. Overall result suggests that diarylpentanoids with both five membered heterocyclic ring and polyhydroxyphenyl moiety could be a new lead design in the search of novel α-glucosidase inhibitor.
中文翻译:

二芳基戊烷系列作为α-葡萄糖苷酶抑制剂的体外和计算机评估
合成了一系列的三十二个二芳基戊烷衍生物,并评估了它们的α-葡萄糖苷酶抑制活性。11种化合物(19,20,21,24,27,28,29,31,32,33和34)被发现显著抑制α葡糖苷酶,其中的化合物28,31和32表现出最高的活性,IC 50值范围从14.1到15.1 µM。结构活性比较表明,多个羟基对于α-葡糖苷酶抑制活性是必不可少的。同时,发现3,4-二羟基苯基和呋喃基部分对于改善α-葡糖苷酶抑制作用至关重要。分子对接分析进一步证实了3,4-二羟基苯基和呋喃基部分在以不同方式与α-葡萄糖苷酶活性位点结合时都起着至关重要的作用。总体结果表明,具有五元杂环和多羟基苯基部分的二芳基戊烷可能是寻找新型α-葡萄糖苷酶抑制剂的一种新的先导设计。


























 京公网安备 11010802027423号
京公网安备 11010802027423号