Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2017-12-24 , DOI: 10.1016/j.bmc.2017.12.035 Edita Čapkauskaitė , Audrius Zakšauskas , Virginijus Ruibys , Vaida Linkuvienė , Vaida Paketurytė , Marius Gedgaudas , Visvaldas Kairys , Daumantas Matulis
|
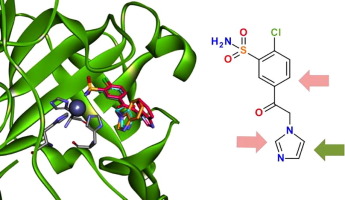
The similarity of human carbonic anhydrase (CA) active sites makes it difficult to design selective inhibitors for one or several CA isoforms that are drug targets. Here we synthesize a series of compounds that are based on 5-[2-(benzimidazol-1-yl)acetyl]-2-chloro-benzenesulfonamide (1a) which demonstrated picomolar binding affinity and significant selectivity for CA isoform five A (VA), and explain the structural influence of inhibitor functional groups to the binding affinity and selectivity. A series of chloro-substituted benzenesulfonamides bearing a heterocyclic tail, together with molecular docking, was used to build inhibitors that explore substituent influence on the binding affinity to the CA VA isoform.
中文翻译:

苯并咪唑的设计,合成和对接以构建选择性碳酸酐酶VA抑制剂
人类碳酸酐酶(CA)活性位点的相似性使得难以设计针对一种或几种作为药物靶标的CA同工型的选择性抑制剂。在这里,我们合成了一系列基于5- [2-(苯并咪唑-1-基)乙酰基] -2-氯-苯磺酰胺(1a)的化合物,这些化合物表现出皮摩尔结合亲和力和对CA亚型5 A(VA)的显着选择性。 ,并解释抑制剂官能团对结合亲和力和选择性的结构影响。一系列带有杂环尾部的氯取代苯磺酰胺与分子对接一起被用于构建抑制剂,该抑制剂探索取代基对与CA VA同工型的结合亲和力的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号