Tetrahedron Letters ( IF 1.8 ) Pub Date : 2017-12-26 , DOI: 10.1016/j.tetlet.2017.12.073 Guangwen Yang , Wentao Wu , Zhixiang Li , Naiwu Chen , Jian Qin , Guoping Hu , Yang Zhang , Jian Li , Shuhui Chen
|
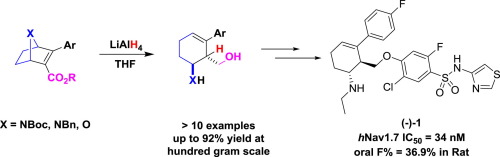
hNav1.7 had received great interest in the past decade owing to its attractive mechanism of actions in pain processing pathway. More recently, we developed a series of efficacious hNav1.7 inhibitors for analgesic, which were characterized with a novel aminocyclohexene as pharmacophore. Herein, we reported our synthetic efforts to construct the unique scaffold, which can be rapidly accessed from readily available 7-azanorbornenes via an unexpected catalyst-free, LiAlH4 mediated tandem double bond migration-ring opening sequence. A probable mechanism was proposed to illuminate the good regio- and stereoselectivity.
中文翻译:

通过LiAlH 4介导的串联双键迁移环开放序列从7-氮杂降冰片有效立体选择性合成氨基环己烯
h Nav1.7由于其在疼痛处理途径中的有吸引力的作用机制而在过去十年中引起了极大的兴趣。最近,我们开发了一系列有效的止痛药h Nav1.7抑制剂,这些抑制剂的特征是使用新型氨基环己烯作为药效基团。在这里,我们报告了我们为构建独特的支架所做的合成努力,可以通过意想不到的无催化剂,LiAlH 4介导的串联双键迁移环打开序列,从容易获得的7-氮杂降冰片中快速获得该支架。提出了一种可能的机制来阐明良好的区域选择性和立体选择性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号