当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Methodology for the Absolute Configuration Determination of Epoxythymols Using the Constituents of Ageratina glabrata
Journal of Natural Products ( IF 5.1 ) Pub Date : 2017-12-26 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00637 Héctor M. Arreaga-González 1 , Julio C. Pardo-Novoa 1 , Rosa E. del Río 1 , Gabriela Rodríguez-García 1 , J. Martín Torres-Valencia 2 , J. Jesús Manríquez-Torres 3 , Carlos M. Cerda-García-Rojas 4 , Pedro Joseph-Nathan 4 , Mario A. Gómez-Hurtado 1
Journal of Natural Products ( IF 5.1 ) Pub Date : 2017-12-26 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00637 Héctor M. Arreaga-González 1 , Julio C. Pardo-Novoa 1 , Rosa E. del Río 1 , Gabriela Rodríguez-García 1 , J. Martín Torres-Valencia 2 , J. Jesús Manríquez-Torres 3 , Carlos M. Cerda-García-Rojas 4 , Pedro Joseph-Nathan 4 , Mario A. Gómez-Hurtado 1
Affiliation
|
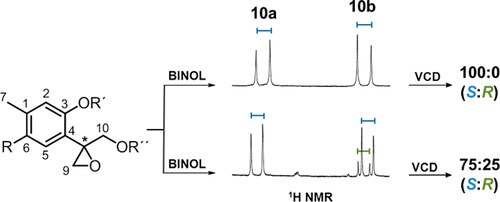
A methodology to determine the enantiomeric excess and the absolute configuration (AC) of natural epoxythymols was developed and tested using five constituents of Ageratina glabrata. The methodology is based on enantiomeric purity determination employing 1,1′-bi-2-naphthol (BINOL) as a chiral solvating agent combined with vibrational circular dichroism (VCD) measurements and calculations. The conformational searching included an extensive Monte Carlo protocol that considered the rotational barriers to cover the whole conformational spaces. (+)-(8S)-10-Benzoyloxy-6-hydroxy-8,9-epoxythymol isobutyrate (1), (+)-(8S)-10-acetoxy-6-methoxy-8,9-epoxythymol isobutyrate (4), and (+)-(8S)-10-benzoyloxy-6-methoxy-8,9-epoxythymol isobutyrate (5) were isolated as enantiomerically pure constituents, while 10-isobutyryloxy-8,9-epoxythymol isobutyrate (2) was obtained as a 75:25 (8S)/(8R) scalemic mixture. In the case of 10-benzoyloxy-8,9-epoxythymol isobutyrate (3), the BINOL methodology revealed a 56:44 scalemic mixture and the VCD measurement was beyond the limit of sensitivity since the enantiomeric excess is only 12%. The racemization process of epoxythymol derivatives was studied using compound 1 and allowed the clarification of some stereochemical aspects of epoxythymol derivatives since their ACs have been scarcely analyzed and a particular behavior in their specific rotations was detected. In more than 30 oxygenated thymol derivatives, including some epoxythymols, the reported specific rotation values fluctuate from −1.6 to +1.4 passing through zero, suggesting the presence of scalemic and close to racemic mixtures, since enantiomerically pure natural constituents showed positive or negative specific rotations greater than 10 units.
中文翻译:

方法论Epoxythymols的绝对构型确定使用的成分紫茎泽兰光滑
开发了一种测定天然环氧麝香草酚的对映异构体过量和绝对构型(AC)的方法,并使用了鼠尾草(Ageratina glabrata)的五种成分进行了测试。该方法基于对映体纯度的确定,该对映体纯度的确定是使用1,1'-联-2-萘酚(BINOL)作为手性溶剂,并结合了振动圆二色性(VCD)进行测量和计算。构象搜索包括广泛的蒙特卡洛协议,该协议考虑了旋转障碍物以覆盖整个构象空间。(+)-(8 S)-10-苯甲酰氧基-6-羟基-8,9-环氧百里酚异丁酸酯(1),(+)-(8 S)-10-乙酰氧基-6-甲氧基-8,9-环氧百里酚异丁酸酯(4)和(+)-(8 S)-10-苯甲酰氧基-6-甲氧基-8,9-环氧百里酚异丁酸酯(5)被分离为对映体纯组分,而10-异丁酰氧基-8,9-环氧百里酚异丁酸酯(2)以75:25(8 S)/(8 R)鳞状混合物。在10-苯甲酰氧基-8,9-环氧百里酚异丁酸酯(3)的情况下,BINOL方法显示出56:44的鳞状混合物,并且VCD测量超出了灵敏度极限,因为对映异构体过量仅为12%。使用化合物1研究环氧百里香酚衍生物的外消旋过程并允许澄清环氧百里香酚衍生物的一些立体化学方面,因为很少对它们的AC进行分析,并且检测到其比旋度中的特定行为。在30多种含氧百里香酚衍生物中,包括一些环氧百里香酚,报告的比旋光度从-1.6到+1.4波动,并通过零波动,这表明存在对映体和接近外消旋混合物,因为对映体纯天然成分显示出正旋向或负旋向。大于10个单位。
更新日期:2017-12-26
中文翻译:

方法论Epoxythymols的绝对构型确定使用的成分紫茎泽兰光滑
开发了一种测定天然环氧麝香草酚的对映异构体过量和绝对构型(AC)的方法,并使用了鼠尾草(Ageratina glabrata)的五种成分进行了测试。该方法基于对映体纯度的确定,该对映体纯度的确定是使用1,1'-联-2-萘酚(BINOL)作为手性溶剂,并结合了振动圆二色性(VCD)进行测量和计算。构象搜索包括广泛的蒙特卡洛协议,该协议考虑了旋转障碍物以覆盖整个构象空间。(+)-(8 S)-10-苯甲酰氧基-6-羟基-8,9-环氧百里酚异丁酸酯(1),(+)-(8 S)-10-乙酰氧基-6-甲氧基-8,9-环氧百里酚异丁酸酯(4)和(+)-(8 S)-10-苯甲酰氧基-6-甲氧基-8,9-环氧百里酚异丁酸酯(5)被分离为对映体纯组分,而10-异丁酰氧基-8,9-环氧百里酚异丁酸酯(2)以75:25(8 S)/(8 R)鳞状混合物。在10-苯甲酰氧基-8,9-环氧百里酚异丁酸酯(3)的情况下,BINOL方法显示出56:44的鳞状混合物,并且VCD测量超出了灵敏度极限,因为对映异构体过量仅为12%。使用化合物1研究环氧百里香酚衍生物的外消旋过程并允许澄清环氧百里香酚衍生物的一些立体化学方面,因为很少对它们的AC进行分析,并且检测到其比旋度中的特定行为。在30多种含氧百里香酚衍生物中,包括一些环氧百里香酚,报告的比旋光度从-1.6到+1.4波动,并通过零波动,这表明存在对映体和接近外消旋混合物,因为对映体纯天然成分显示出正旋向或负旋向。大于10个单位。


























 京公网安备 11010802027423号
京公网安备 11010802027423号