Biochimie ( IF 3.9 ) Pub Date : 2017-12-24 , DOI: 10.1016/j.biochi.2017.12.007 Rui Yang , Guojun Zhang , Furui Zhang , Zhanjiang Li , Chun Huang
|
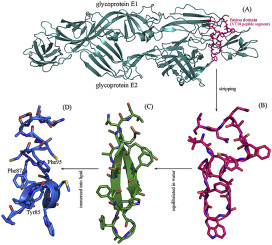
The structural dynamics of membrane permeabilization are investigated systematically and compared between viral fusion peptides (VFPs) and antimicrobial peptides (AMPs). It is revealed that the permeabilization process can be divided into two phases: a fast motion phase in water (first phase) and a slow diffusion phase in lipid (second phase). Difference in peptide permeability to neutrally or weakly charged mammalian membrane and to negatively charged bacterial membrane is primarily determined by the first phase, which is dominated by the direct electrostatic interaction between peptide and the hydrophilic surface of membranes. With the harvested knowledge we attempt to rationally design anti-Gram-positive AMPs based on the VFP scaffold of Chikungunya virus fusion domain, which is an 18-mer polypeptide segment (VT18, 84VYPFMWGGAYCFCDAENT101) located in the structural glycoprotein E1 of viral envelope. Our simulations and previous NMR study suggest that the isolated VT18 peptide can be well structured into a double-stranded β-sheet conformation in water, but would become intrinsically disordering in lipid. Converting the negatively charged VT18 (charge = −2) to two positively charged peptides VT18-KKLV (VYPFMWGGAYCFCKAKLV-NH2) (charge = +3) and VT18-CAKKLV (VYPFCWGGAYAFCKAKLV-NH2) (charge = +3) by residue substitution and C-terminal amidation can largely promote peptide approaching to bacterial membrane surface, thus rendering the peptide with a substantially increased antibacterial activity against Gram-positive Streptococcus pneumoniae (MIC changes from >200 to 52–105 and 58–90 μg/ml, respectively). A further cyclization of linear peptide VT18-CAKKLV by adding a disulfide bond across its two strand arms, which results in a cyclic peptide cVT18-CAKKLV (
中文翻译:

基于基孔肯雅病毒融合域支架的抗菌肽膜通透性设计及其对呼吸道感染革兰氏阳性肺炎链球菌的抗菌活性
系统地研究了膜通透性的结构动力学,并在病毒融合肽(VFP)和抗菌肽(AMP)之间进行了比较。揭示了透化过程可分为两个阶段:在水中的快速运动阶段(第一阶段)和在脂质中的缓慢扩散阶段(第二阶段)。肽对带中性或弱电荷的哺乳动物膜和带负电荷的细菌膜的通透性差异主要由第一阶段决定,该阶段主要由肽与膜的亲水性表面之间的直接静电相互作用所决定。与收获的知识,我们尝试合理设计基于屈曲病毒融合结构域的VFP脚手架,这是一个18聚体多肽片段的抗革兰氏阳性的AMP(VT18,84VYPFMWGGAYCFCDAENT 101)位于病毒包膜的结构糖蛋白E1中。我们的模拟和先前的NMR研究表明,分离出的VT18肽在水中可以很好地构建为双链β-折叠构象,但在脂质中会固有地无序。通过残基取代将带负电荷的VT18(电荷= -2)转换为两个带正电荷的肽VT18-KKLV(VYPFMWGGAYCFCKAKLV-NH 2)(电荷= +3)和VT18-CAKKLV(VYPFCWGGAYAFCKAKLV-NH 2)(电荷= +3) C末端酰胺化可大大促进肽接近细菌膜表面,从而使该肽对革兰氏阳性菌的抗菌活性大大提高肺炎链球菌(MIC分别从> 200降至52–105和58–90μg/ ml)。通过在线性肽VT18-CAKKLV的两个链臂之间添加一个二硫键,进一步环化,从而形成环状肽cVT18-CAKKLV(




























 京公网安备 11010802027423号
京公网安备 11010802027423号