当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Structural and Functional Role for Disulfide Bonds in a Class II Hydrophobin
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acs.biochem.7b01166 Nathanael D. Sallada 1 , Kerri J. Dunn 2 , Bryan W. Berger 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acs.biochem.7b01166 Nathanael D. Sallada 1 , Kerri J. Dunn 2 , Bryan W. Berger 1, 2
Affiliation
|
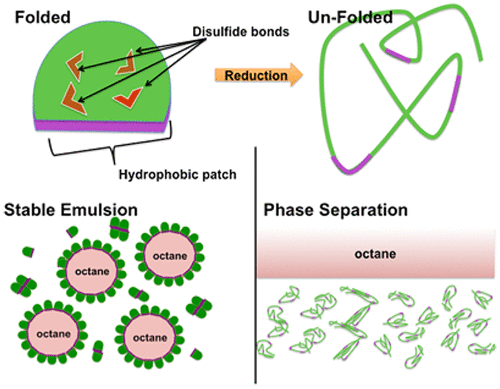
Hydrophobins are multifunctional, highly surface active proteins produced in filamentous fungi and can be identified by eight conserved cysteine residues, which form four disulfide bridges. These proteins can be subdivided into two classes based on their hydropathy profiles, solubility, and structures formed upon interfacial assembly. Here, we probe the structural and functional roles of disulfide bonds for a class II hydrophobin in different interfacial contexts by reducing its disulfides with 1,4-dithiothreitol and blocking the free thiols with iodoacetamide and then examining the protein secondary structure, emulsification capability, hydrophobic surface wetting, and solution self-assembly. Changes in circular dichroism spectra upon reduction and blocking of disulfides are consistent with an increase in the level of random coil secondary structure. Emulsification of octane in water using reduced and unreduced forms of class II hydrophobin showed a substantial loss of emulsification ability without disulfides and stable emulsion formation for hydrophobin with disulfides. Additionally, water contact angle measurements performed on polytetrafluoroethylene treated with solutions of reduced and unreduced hydrophobin showed efficient wetting of the hydrophobic surface for unreduced samples only. Lastly, Förster resonance energy transfer (FRET) was used to assess the role of disulfides in self-assembly in solution, and near complete loss of the FRET signal is consistent with a model in which solution self-assembly does not occur after reduction and blocking of the disulfides. From this, we conclude that, in contrast to class I hydrophobins, the disulfides of this class II hydrophobin are required for protein structural stability, surface activity at both liquid–liquid and solid–liquid interfaces, and solution self-assembly.
中文翻译:

II类疏水蛋白中二硫键的结构和功能作用
疏水蛋白是在丝状真菌中产生的多功能,高度表面活性的蛋白,可以通过八个保守的半胱氨酸残基鉴定,这些残基形成四个二硫键。根据它们的亲水性,溶解度和在界面组装时形成的结构,这些蛋白质可分为两类。在这里,我们通过用1,4-二硫苏糖醇还原二硫键并用碘乙酰胺封闭游离硫醇,然后研究蛋白质的二级结构,乳化能力,疏水性,来探讨二硫键在不同界面环境中二硫键的结构和功能作用表面润湿和溶液自组装。二硫化物的还原和封闭后,圆二色性光谱的变化与无规卷曲二级结构水平的增加是一致的。使用还原和未还原形式的II类疏水蛋白在水中进行辛烷值的乳化显示,没有二硫化物的乳化能力会大大降低,并且对于具有二硫化物的疏水蛋白,乳液的形成也很稳定。另外,在用还原的和未还原的疏水蛋白溶液处理的聚四氟乙烯上进行的水接触角测量显示,仅对于未还原的样品,疏水表面的有效润湿。最后,使用Förster共振能量转移(FRET)来评估二硫化物在溶液中自组装中的作用,FRET信号几乎完全丧失,与其中二硫化物还原和封闭后不会发生溶液自组装的模型相一致。由此,我们得出结论,与I类疏水蛋白相反,II类疏水蛋白的二硫键是蛋白质结构稳定性,液-液和固-液界面的表面活性以及溶液自组装所必需的。
更新日期:2018-01-09
中文翻译:

II类疏水蛋白中二硫键的结构和功能作用
疏水蛋白是在丝状真菌中产生的多功能,高度表面活性的蛋白,可以通过八个保守的半胱氨酸残基鉴定,这些残基形成四个二硫键。根据它们的亲水性,溶解度和在界面组装时形成的结构,这些蛋白质可分为两类。在这里,我们通过用1,4-二硫苏糖醇还原二硫键并用碘乙酰胺封闭游离硫醇,然后研究蛋白质的二级结构,乳化能力,疏水性,来探讨二硫键在不同界面环境中二硫键的结构和功能作用表面润湿和溶液自组装。二硫化物的还原和封闭后,圆二色性光谱的变化与无规卷曲二级结构水平的增加是一致的。使用还原和未还原形式的II类疏水蛋白在水中进行辛烷值的乳化显示,没有二硫化物的乳化能力会大大降低,并且对于具有二硫化物的疏水蛋白,乳液的形成也很稳定。另外,在用还原的和未还原的疏水蛋白溶液处理的聚四氟乙烯上进行的水接触角测量显示,仅对于未还原的样品,疏水表面的有效润湿。最后,使用Förster共振能量转移(FRET)来评估二硫化物在溶液中自组装中的作用,FRET信号几乎完全丧失,与其中二硫化物还原和封闭后不会发生溶液自组装的模型相一致。由此,我们得出结论,与I类疏水蛋白相反,II类疏水蛋白的二硫键是蛋白质结构稳定性,液-液和固-液界面的表面活性以及溶液自组装所必需的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号