当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Quintuple [6]Helicene with a Corannulene Core as a C5‐Symmetric Propeller‐Shaped π‐System
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-01-09 , DOI: 10.1002/anie.201711985 Kenta Kato 1 , Yasutomo Segawa 1, 2 , Lawrence T. Scott 3 , Kenichiro Itami 1, 2, 4
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-01-09 , DOI: 10.1002/anie.201711985 Kenta Kato 1 , Yasutomo Segawa 1, 2 , Lawrence T. Scott 3 , Kenichiro Itami 1, 2, 4
Affiliation
|
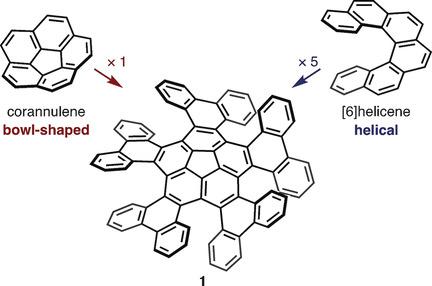
The synthesis and structural analysis of a quintuple [6]helicene with a corannulene core is reported. The compound was synthesized from corannulene in three steps including a five‐fold intramolecular direct arylation. X‐ray crystallographic analysis revealed a C5‐symmetric propeller‐shaped structure and one‐dimensional alignment in the solid state. The enantiomers of the quintuple [6]helicene were successfully separated by HPLC, and the chirality of the two fractions was identified by CD spectroscopy. A kinetic study yielded a racemization barrier of 34.2 kcal mol−1, which is slightly lower than that of pristine [6]helicene. DFT calculations indicate a rapid bowl‐to‐bowl inversion of the corannulene moiety and a step‐by‐step chiral inversion pathway for the five [6]helicene moieties.
中文翻译:

具有Corannulene核心的五元组[6]螺旋形螺帽,作为C5对称螺旋桨形π系统
报道了具有氢化萘芯的五元[6]螺旋烯的合成和结构分析。该化合物是通过三步合成的,由Corannulene合成,包括五倍的分子内直接芳基化。X射线晶体学分析表明,C 5对称的螺旋桨状结构和固态一维排列。通过HPLC成功分离了五元组[6]螺旋烯的对映异构体,并通过CD光谱法鉴定了这两个馏分的手性。动力学研究得出消旋化势垒为34.2 kcal mol -1,比原始的[6]螺旋烯稍低。DFT计算表明,对五个[6]螺旋烯部分,Corannulene部分具有快速的碗对碗倒置和逐步的手性倒置路径。
更新日期:2018-01-09
中文翻译:

具有Corannulene核心的五元组[6]螺旋形螺帽,作为C5对称螺旋桨形π系统
报道了具有氢化萘芯的五元[6]螺旋烯的合成和结构分析。该化合物是通过三步合成的,由Corannulene合成,包括五倍的分子内直接芳基化。X射线晶体学分析表明,C 5对称的螺旋桨状结构和固态一维排列。通过HPLC成功分离了五元组[6]螺旋烯的对映异构体,并通过CD光谱法鉴定了这两个馏分的手性。动力学研究得出消旋化势垒为34.2 kcal mol -1,比原始的[6]螺旋烯稍低。DFT计算表明,对五个[6]螺旋烯部分,Corannulene部分具有快速的碗对碗倒置和逐步的手性倒置路径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号