当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereoselective Construction of Halogenated Quaternary Carbon Centers by Brønsted Base Catalyzed [4+2] Cycloaddition of α‐Haloaldehydes
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-01-16 , DOI: 10.1002/anie.201711813 Qiang Li 1 , Liang Zhou 1 , Xu-Dong Shen 1 , Kai-Chuan Yang 1 , Xiang Zhang 2 , Qing-Song Dai 1 , Hai-Jun Leng 1 , Qing-Zhu Li 1 , Jun-Long Li 1, 2
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-01-16 , DOI: 10.1002/anie.201711813 Qiang Li 1 , Liang Zhou 1 , Xu-Dong Shen 1 , Kai-Chuan Yang 1 , Xiang Zhang 2 , Qing-Song Dai 1 , Hai-Jun Leng 1 , Qing-Zhu Li 1 , Jun-Long Li 1, 2
Affiliation
|
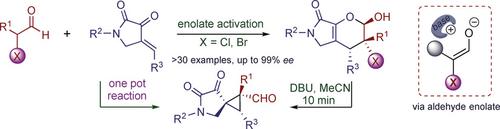
Asymmetric construction of halogenated quaternary carbon centers under mild reaction conditions remains challenging. Reported here is an unprecedented and highly stereoselective Brønsted base catalyzed [4+2] cycloaddition between either α‐chloro‐ or α‐bromoaldehydes and cyclic enones. The key intermediate, an α‐halogenated enolate, is susceptible to dehalogenation and can be stabilized and stereochemically controlled using bifunctional tertiary amines. This method provides facile access to a collection of optically pure bicyclic dihydropyrans having three contiguous stereocenters, including a halogen‐bearing quaternary carbon center. Of note, the product can be transformed in situ into densely functionalized spirocyclopropanes in a highly efficient and stereoselective manner.
中文翻译:

布朗斯台德碱催化α-卤代醛的[4 + 2]环加成反应立体构筑卤代季碳中心
在温和的反应条件下卤代季碳中心的不对称结构仍然具有挑战性。此处报道的是α-氯醛或α-溴醛与环状烯酮之间空前的,高度立体选择性的布朗斯台德碱催化的[4 + 2]环加成反应。关键中间体是α-卤代烯醇化物,易于脱卤,可以使用双官能叔胺稳定和进行立体化学控制。该方法可轻松访问具有三个连续立体中心(包括一个含卤素的季碳中心)的光学纯双环二氢吡喃类化合物。值得注意的是,该产物可以以高效和立体选择性的方式原位转化为高密度官能化的螺环丙烷。
更新日期:2018-01-16
中文翻译:

布朗斯台德碱催化α-卤代醛的[4 + 2]环加成反应立体构筑卤代季碳中心
在温和的反应条件下卤代季碳中心的不对称结构仍然具有挑战性。此处报道的是α-氯醛或α-溴醛与环状烯酮之间空前的,高度立体选择性的布朗斯台德碱催化的[4 + 2]环加成反应。关键中间体是α-卤代烯醇化物,易于脱卤,可以使用双官能叔胺稳定和进行立体化学控制。该方法可轻松访问具有三个连续立体中心(包括一个含卤素的季碳中心)的光学纯双环二氢吡喃类化合物。值得注意的是,该产物可以以高效和立体选择性的方式原位转化为高密度官能化的螺环丙烷。


























 京公网安备 11010802027423号
京公网安备 11010802027423号