当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic Model for the Conversion of Chloromethane into Hydrocarbons over a HZSM-5 Zeolite Catalyst
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.iecr.7b04379 Monica Gamero 1 , Beatriz Valle 1 , Ana G. Gayubo 1 , Pedro Castaño 1 , Andres T. Aguayo 1 , Javier Bilbao 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.iecr.7b04379 Monica Gamero 1 , Beatriz Valle 1 , Ana G. Gayubo 1 , Pedro Castaño 1 , Andres T. Aguayo 1 , Javier Bilbao 1
Affiliation
|
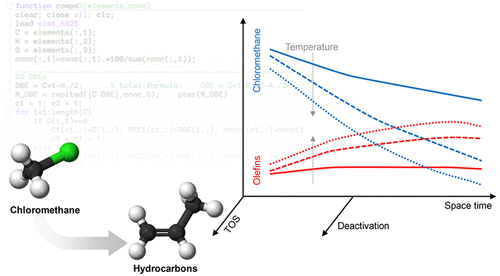
The conversion of chloromethane into hydrocarbons over a HZSM-5 zeolite based catalyst is investigated with the aim of establishing a kinetic model suitable for simulating this process for indirect valorization of the methane contained in shale gas. The experimental data were obtained in an isothermal fixed bed reactor under a wide range of operating conditions: temperature, 300–450 °C; space-time, 1–12 gcatalyst h/molCH2; chloromethane partial pressure, 0.375–1.5 atm; and time on stream, up to 255 min. The reaction scheme is composed of eight components and lumps (chloromethane, C2–C4 olefins, C2–C4 paraffins, C5–C10 aliphatics, aromatics BTX, methane, hydrochloric acid, and chlorinated hydrocarbons), which are involved in ten single reactions. The catalyst deactivation by coke deposition has been quantified by an equation, which is dependent on the concentration of chloromethane in the reaction medium. The kinetic model is suitable for predicting accurately the effect that reaction conditions have on products distribution and their evolution with time on stream.
中文翻译:

HZSM-5沸石催化剂上氯甲烷转化为烃的动力学模型
研究了HZSM-5沸石基催化剂上氯甲烷向烃类的转化,目的是建立适用于模拟此过程的动态模型的动力学模型,以间接评估页岩气中所含甲烷的价值。实验数据是在等温固定床反应器中的各种操作条件下获得的:温度为300–450°C;温度为300-450°C。时空,1–12 g催化剂h / mol CH 2;氯甲烷分压,0.375–1.5大气压;和播放时间,最多255分钟。反应方案由八种成分和团块组成(氯甲烷,C 2 -C 4烯烃,C 2 -C 4链烷烃,C 5 -C 10脂族,芳族BTX,甲烷,盐酸和氯代烃),它们涉及十个单一反应。焦炭沉积引起的催化剂失活已经通过方程式进行了定量,该方程式取决于反应介质中氯甲烷的浓度。动力学模型适用于准确预测反应条件对产物分布及其随时间变化的影响。
更新日期:2018-01-12
中文翻译:

HZSM-5沸石催化剂上氯甲烷转化为烃的动力学模型
研究了HZSM-5沸石基催化剂上氯甲烷向烃类的转化,目的是建立适用于模拟此过程的动态模型的动力学模型,以间接评估页岩气中所含甲烷的价值。实验数据是在等温固定床反应器中的各种操作条件下获得的:温度为300–450°C;温度为300-450°C。时空,1–12 g催化剂h / mol CH 2;氯甲烷分压,0.375–1.5大气压;和播放时间,最多255分钟。反应方案由八种成分和团块组成(氯甲烷,C 2 -C 4烯烃,C 2 -C 4链烷烃,C 5 -C 10脂族,芳族BTX,甲烷,盐酸和氯代烃),它们涉及十个单一反应。焦炭沉积引起的催化剂失活已经通过方程式进行了定量,该方程式取决于反应介质中氯甲烷的浓度。动力学模型适用于准确预测反应条件对产物分布及其随时间变化的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号