当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Target Identification and Mode of Action of Four Chemically Divergent Drugs against Ebolavirus Infection.
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2018-01-16 , DOI: 10.1021/acs.jmedchem.7b01249 Jingshan Ren 1 , Yuguang Zhao 1 , Elizabeth E Fry 1 , David I Stuart 1, 2
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2018-01-16 , DOI: 10.1021/acs.jmedchem.7b01249 Jingshan Ren 1 , Yuguang Zhao 1 , Elizabeth E Fry 1 , David I Stuart 1, 2
Affiliation
|
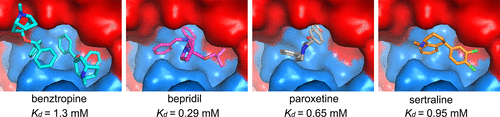
Here, we show that four chemically divergent approved drugs reported to inhibit Ebolavirus infection, benztropine, bepridil, paroxetine and sertraline, directly interact with the Ebolavirus glycoprotein. Binding of these drugs destabilizes the protein, suggesting that this may be the mechanism of inhibition, as reported for the anticancer drug toremifene and the painkiller ibuprofen, which bind in the same large cavity on the glycoprotein. Crystal structures show that the position of binding and the mode of interaction within the pocket vary significantly between these compounds. The binding constants (Kd) determined by thermal shift assay correlate with the protein-inhibitor interactions as well as with the antiviral activities determined by virus cell entry assays, supporting the hypothesis that these drugs inhibit viral entry by binding the glycoprotein and destabilizing the prefusion conformation. Details of the protein-inhibitor interactions of these complexes and their relation with binding affinity may facilitate the design of more potent inhibitors.
中文翻译:

四种化学差异药物对抗埃博拉病毒感染的靶点鉴定和作用方式。
在这里,我们展示了四种化学上不同的获批药物,据报道可抑制埃博拉病毒感染,苯托品、bepridil、帕罗西汀和舍曲林,直接与埃博拉病毒糖蛋白相互作用。这些药物的结合使蛋白质不稳定,这表明这可能是抑制机制,正如抗癌药托瑞米芬和止痛药布洛芬所报道的那样,它们结合在糖蛋白上的同一个大空腔中。晶体结构表明,这些化合物之间的结合位置和口袋内的相互作用模式显着不同。热位移测定法测定的结合常数 (Kd) 与蛋白质-抑制剂相互作用以及病毒细胞进入测定法测定的抗病毒活性相关,支持这些药物通过结合糖蛋白和破坏融合前构象来抑制病毒进入的假设。这些复合物的蛋白质-抑制剂相互作用的细节及其与结合亲和力的关系可能有助于设计更有效的抑制剂。
更新日期:2018-01-16
中文翻译:

四种化学差异药物对抗埃博拉病毒感染的靶点鉴定和作用方式。
在这里,我们展示了四种化学上不同的获批药物,据报道可抑制埃博拉病毒感染,苯托品、bepridil、帕罗西汀和舍曲林,直接与埃博拉病毒糖蛋白相互作用。这些药物的结合使蛋白质不稳定,这表明这可能是抑制机制,正如抗癌药托瑞米芬和止痛药布洛芬所报道的那样,它们结合在糖蛋白上的同一个大空腔中。晶体结构表明,这些化合物之间的结合位置和口袋内的相互作用模式显着不同。热位移测定法测定的结合常数 (Kd) 与蛋白质-抑制剂相互作用以及病毒细胞进入测定法测定的抗病毒活性相关,支持这些药物通过结合糖蛋白和破坏融合前构象来抑制病毒进入的假设。这些复合物的蛋白质-抑制剂相互作用的细节及其与结合亲和力的关系可能有助于设计更有效的抑制剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号