当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Non‐Competitive Inhibitor of VCP/p97 and VPS4 Reveals Conserved Allosteric Circuits in Type I and II AAA ATPases
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-01-15 , DOI: 10.1002/anie.201711429 Robert Pöhler 1 , Jan H. Krahn 2 , Johannes van den Boom 1 , Grzegorz Dobrynin 1 , Farnusch Kaschani 2 , Hans-Michael Eggenweiler 3 , Frank T. Zenke 3 , Markus Kaiser 2 , Hemmo Meyer 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-01-15 , DOI: 10.1002/anie.201711429 Robert Pöhler 1 , Jan H. Krahn 2 , Johannes van den Boom 1 , Grzegorz Dobrynin 1 , Farnusch Kaschani 2 , Hans-Michael Eggenweiler 3 , Frank T. Zenke 3 , Markus Kaiser 2 , Hemmo Meyer 1
Affiliation
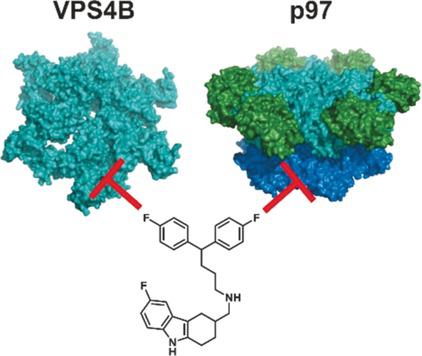
|
AAA ATPases have pivotal functions in diverse cellular processes essential for survival and proliferation. Revealing strategies for chemical inhibition of this class of enzymes is therefore of great interest for the development of novel chemotherapies or chemical tools. Here, we characterize the compound MSC1094308 as a reversible, allosteric inhibitor of the type II AAA ATPase human ubiquitin‐directed unfoldase (VCP)/p97 and the type I AAA ATPase VPS4B. Subsequent proteomic, genetic and biochemical studies indicate that MSC1094308 binds to a previously characterized drugable hotspot of p97, thereby inhibiting the D2 ATPase activity. Our results furthermore indicate that a similar allosteric site exists in VPS4B, suggesting conserved allosteric circuits and drugable sites in both type I and II AAA ATPases. Our results may thus guide future chemical tool and drug discovery efforts for the biomedically relevant AAA ATPases.
中文翻译:

VCP / p97和VPS4的非竞争性抑制剂可揭示I型和II型AAA ATPase的保守变构回路。
AAA ATPase在生存和增殖必不可少的多种细胞过程中具有关键功能。因此,对于这类酶的化学抑制的揭示策略对于新型化学疗法或化学工具的开发非常感兴趣。在这里,我们将化合物MSC1094308表征为II型AAA ATPase人泛素定向解折叠酶(VCP)/ p97和I型AAA ATPase VPS4B的可逆变构抑制剂。随后的蛋白质组学,遗传和生化研究表明,MSC1094308与先前鉴定的p97可药用热点结合,从而抑制了D2 ATPase的活性。我们的结果进一步表明,VPS4B中存在类似的变构位点,这表明在I型和II型AAA ATP酶中均存在保守的变构回路和可药用位点。
更新日期:2018-01-15
中文翻译:

VCP / p97和VPS4的非竞争性抑制剂可揭示I型和II型AAA ATPase的保守变构回路。
AAA ATPase在生存和增殖必不可少的多种细胞过程中具有关键功能。因此,对于这类酶的化学抑制的揭示策略对于新型化学疗法或化学工具的开发非常感兴趣。在这里,我们将化合物MSC1094308表征为II型AAA ATPase人泛素定向解折叠酶(VCP)/ p97和I型AAA ATPase VPS4B的可逆变构抑制剂。随后的蛋白质组学,遗传和生化研究表明,MSC1094308与先前鉴定的p97可药用热点结合,从而抑制了D2 ATPase的活性。我们的结果进一步表明,VPS4B中存在类似的变构位点,这表明在I型和II型AAA ATP酶中均存在保守的变构回路和可药用位点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号