当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selenite Uptake by Ca–Al LDH: A Description of Intercalated Anion Coordination Geometries
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.est.7b04644 Bin Ma 1 , Alejandro Fernandez-Martinez 1 , Sylvain Grangeon 2 , Christophe Tournassat 2, 3, 4 , Nathaniel Findling 1 , Sergio Carrero 5, 6 , Delphine Tisserand 1 , Sarah Bureau 1 , Erik Elkaïm 7 , Carlo Marini 8 , Giuliana Aquilanti 9 , Ayumi Koishi 1 , Nicolas C. M. Marty 2 , Laurent Charlet 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.est.7b04644 Bin Ma 1 , Alejandro Fernandez-Martinez 1 , Sylvain Grangeon 2 , Christophe Tournassat 2, 3, 4 , Nathaniel Findling 1 , Sergio Carrero 5, 6 , Delphine Tisserand 1 , Sarah Bureau 1 , Erik Elkaïm 7 , Carlo Marini 8 , Giuliana Aquilanti 9 , Ayumi Koishi 1 , Nicolas C. M. Marty 2 , Laurent Charlet 1
Affiliation
|
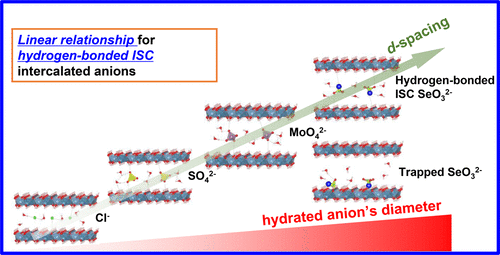
Layered double hydroxides (LDHs) are anion exchangers with a strong potential to scavenge anionic contaminants in aquatic environments. Here, the uptake of selenite (SeO32–) by Ca–Al LDHs was investigated as a function of Se concentration. Thermodynamic modeling of batch sorption isotherms shows that the formation of SeO32–-intercalated AFm (hydrated calcium aluminate monosubstituent) phase, AFm-SeO3, is the dominant mechanism controlling the retention of Se at medium loadings. AFm-Cl2 shows much stronger affinity and larger distribution ratio (Rd ∼ 17800 L kg–1) toward SeO32– than AFm-SO4 (Rd ∼ 705 L kg–1). At stoichiometric SeO32– loading for anion exchange, the newly formed AFm-SeO3 phase results in two basal spacing, i.e., 9.93 ± 0.06 Å and ∼11.03 ± 0.03 Å. Extended X-ray absorption fine structure (EXAFS) spectra indicate that the intercalated SeO32– forms inner-sphere complexes with the Ca–Al–O layers. In situ X-ray diffraction (XRD) shows that basal spacing of Ca–Al LDHs have a remarkable linear relationship with the size of hydrated intercalated anions (i.e., Cl–, SO42–, MoO42–, and SeO32–). Contrary to AFm-SeO3 with inner-sphere SeO32– complexes in the interlayer, the phase with hydrogen-bonded inner-sphere complexed SeO32– is kinetically favored but thermodynamically unstable. This work offers new insights about the determination of intercalated anion coordination geometries via XRD analyses.
中文翻译:

Ca-Al LDH对亚硒酸盐的吸收:插层阴离子配位几何的描述
层状双氢氧化物(LDH)是阴离子交换剂,具有清除水生环境中阴离子污染物的强大潜力。在这里,研究了Ca-Al LDHs对硒(SeO 3 2-)的吸收与硒浓度的关系。批量吸附等温线的热力学模型表明,SeO 3 2-插层的AFm(水合铝酸钙单取代物)相AFm-SeO 3的形成是控制Se在中等负荷下保留的主要机制。AFM-CL 2示出了强得多的亲和力,以及较大的分配比([R d〜17800公斤大号-1)的SeO朝向3 2-比AFM-SO 4(- [R d〜705公斤大号-1)。在用于阴离子交换的化学计量的SeO 3 2-负载下,新形成的AFm-SeO 3相产生两个基本间距,即9.93±0.06Å和〜11.03±0.03Å。扩展的X射线吸收精细结构(EXAFS)光谱表明,嵌入的SeO 3 2–与Ca–Al–O层形成内球络合物。原位X射线衍射(XRD)表明,Ca–Al LDH的基本间距与水合插层阴离子(即Cl –,SO 4 2–,MoO 4 2–和SeO 3 2)的大小具有显着的线性关系。–)。与在中间层中带有内球SeO 3 2 –配合物的AFm-SeO 3相反,具有氢键结合内球的SeO 3 2–的相在动力学上是有利的,但在热力学上是不稳定的。这项工作为通过XRD分析确定插层阴离子配位几何提供了新的见解。
更新日期:2018-01-11
中文翻译:

Ca-Al LDH对亚硒酸盐的吸收:插层阴离子配位几何的描述
层状双氢氧化物(LDH)是阴离子交换剂,具有清除水生环境中阴离子污染物的强大潜力。在这里,研究了Ca-Al LDHs对硒(SeO 3 2-)的吸收与硒浓度的关系。批量吸附等温线的热力学模型表明,SeO 3 2-插层的AFm(水合铝酸钙单取代物)相AFm-SeO 3的形成是控制Se在中等负荷下保留的主要机制。AFM-CL 2示出了强得多的亲和力,以及较大的分配比([R d〜17800公斤大号-1)的SeO朝向3 2-比AFM-SO 4(- [R d〜705公斤大号-1)。在用于阴离子交换的化学计量的SeO 3 2-负载下,新形成的AFm-SeO 3相产生两个基本间距,即9.93±0.06Å和〜11.03±0.03Å。扩展的X射线吸收精细结构(EXAFS)光谱表明,嵌入的SeO 3 2–与Ca–Al–O层形成内球络合物。原位X射线衍射(XRD)表明,Ca–Al LDH的基本间距与水合插层阴离子(即Cl –,SO 4 2–,MoO 4 2–和SeO 3 2)的大小具有显着的线性关系。–)。与在中间层中带有内球SeO 3 2 –配合物的AFm-SeO 3相反,具有氢键结合内球的SeO 3 2–的相在动力学上是有利的,但在热力学上是不稳定的。这项工作为通过XRD分析确定插层阴离子配位几何提供了新的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号