当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Controllable Generation of Free Radicals from Multifunctional Heat-Responsive Nanoplatform for Targeted Cancer Therapy
Chemistry of Materials ( IF 8.6 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.chemmater.7b04841 Lili Feng 1 , Shili Gai 1 , Yunlu Dai 1 , Fei He 1 , Chunqiang Sun 2 , Piaoping Yang 1 , Ruichan Lv 3 , Na Niu 4 , Guanghui An 1 , Jun Lin 2
Chemistry of Materials ( IF 8.6 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.chemmater.7b04841 Lili Feng 1 , Shili Gai 1 , Yunlu Dai 1 , Fei He 1 , Chunqiang Sun 2 , Piaoping Yang 1 , Ruichan Lv 3 , Na Niu 4 , Guanghui An 1 , Jun Lin 2
Affiliation
|
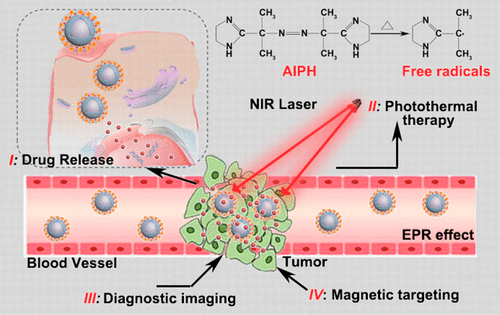
Targeted drug delivery and controllable generation of oxygen-independent toxic free radicals in tumor hypoxia environments are of great importance in cancer therapy. Here, a thermoresponsive nanoplatform was introduced by modifying carbon-coated iron carbide (Fe5C2) nanoparticles with bovine serum albumin (BSA) to achieve better water solubility and biocompatibility. Then a type of polymerization initiator (AIPH) was effectively loaded on the surface of Fe5C2–BSA nanoparticles and sealed by a phase change material (PCM) for higher drug loading and controlled drug release stimulated by heat. Upon illumination by near-infrared light, the photothermal effect of Fe5C2 nanoparticles melts the PCM, triggering the release of encapsulated AIPH to produce free radicals, which effectively kill the hypoxia cancer cells. Additionally, the special magnetic performance enables targeted and tracked therapy under the driving of an external magnetic field. What’s more, the as-prepared multifunctional theranostic nanoplatform (Fe5C2–BSA-AIPH/PCM) ingeniously combine magnetic targeting, remotely controlled drug delivery, the generation of free radicals independent of oxygen levels into a single nanoparticle for effective cancer treatment, in detail, causing cancer cells death in vitro and markedly inhibiting tumor growth in vivo. This work presents a paradigm demonstrating that enhancing the therapeutic effect based on rationally designed multifunctional nanotheranostic agent will pioneer a new way for synergistic cancer treatment and highly developing nanotechnology.
中文翻译:

从多功能热响应纳米平台可控的自由基靶向治疗癌症的产生。
在肿瘤缺氧环境中靶向药物递送和氧非依赖性毒性自由基的可控生成在癌症治疗中非常重要。在这里,通过用牛血清白蛋白(BSA)修饰碳包覆的碳化铁(Fe 5 C 2)纳米颗粒,引入了热响应性纳米平台,以实现更好的水溶性和生物相容性。然后将一种类型的聚合引发剂(AIPH)有效地负载在Fe 5 C 2 -BSA纳米颗粒的表面上,并用相变材料(PCM)密封,以增加载药量并控制受热刺激的药物释放。在近红外光照射下,Fe 5 C 2的光热效应纳米粒子会熔化PCM,从而触发封装的AIPH释放以产生自由基,从而有效杀死低氧癌细胞。此外,特殊的磁性能可以在外部磁场的驱动下进行有针对性的跟踪治疗。此外,所制备的多功能治疗药物纳米平台(Fe 5 C 2–BSA-AIPH / PCM)巧妙地将磁性靶向,远程控制药物输送,与氧水平无关的自由基的生成结合到单个纳米颗粒中,以进行有效的癌症治疗,详细地讲,该方法可导致癌细胞在体外死亡并显着抑制肿瘤的生长。体内。这项工作提出了一个范式,表明基于合理设计的多功能纳米治疗剂增强治疗效果将开创一种协同治疗癌症和高度发展的纳米技术的新方法。
更新日期:2018-01-08
中文翻译:

从多功能热响应纳米平台可控的自由基靶向治疗癌症的产生。
在肿瘤缺氧环境中靶向药物递送和氧非依赖性毒性自由基的可控生成在癌症治疗中非常重要。在这里,通过用牛血清白蛋白(BSA)修饰碳包覆的碳化铁(Fe 5 C 2)纳米颗粒,引入了热响应性纳米平台,以实现更好的水溶性和生物相容性。然后将一种类型的聚合引发剂(AIPH)有效地负载在Fe 5 C 2 -BSA纳米颗粒的表面上,并用相变材料(PCM)密封,以增加载药量并控制受热刺激的药物释放。在近红外光照射下,Fe 5 C 2的光热效应纳米粒子会熔化PCM,从而触发封装的AIPH释放以产生自由基,从而有效杀死低氧癌细胞。此外,特殊的磁性能可以在外部磁场的驱动下进行有针对性的跟踪治疗。此外,所制备的多功能治疗药物纳米平台(Fe 5 C 2–BSA-AIPH / PCM)巧妙地将磁性靶向,远程控制药物输送,与氧水平无关的自由基的生成结合到单个纳米颗粒中,以进行有效的癌症治疗,详细地讲,该方法可导致癌细胞在体外死亡并显着抑制肿瘤的生长。体内。这项工作提出了一个范式,表明基于合理设计的多功能纳米治疗剂增强治疗效果将开创一种协同治疗癌症和高度发展的纳米技术的新方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号