当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A nickel-catalyzed anti-carbometallative cyclization of alkyne–azides with organoboronic acids: synthesis of 2,3-diarylquinolines
Chemical Communications ( IF 4.9 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1039/c7cc08408k Gadi Ranjith Kumar 1, 2, 3, 4, 5 , Ravi Kumar 1, 2, 3, 4, 5 , Manda Rajesh 1, 2, 3, 4 , Maddi Sridhar Reddy 1, 2, 3, 4, 5
Chemical Communications ( IF 4.9 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1039/c7cc08408k Gadi Ranjith Kumar 1, 2, 3, 4, 5 , Ravi Kumar 1, 2, 3, 4, 5 , Manda Rajesh 1, 2, 3, 4 , Maddi Sridhar Reddy 1, 2, 3, 4, 5
Affiliation
|
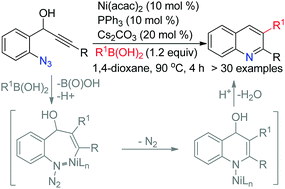
An anti-carbonickelative cyclization via reversible alkenylnickel E/Z isomerization of 2-azido phenyl propargyl alcohols with aryl boronic acids is achieved using Ni(acac)2 as the catalyst to access 2,3-diaryl quinolines. It represents a rare example of trapping the vinyl metal intermediate with a nitrogen center, a non-carbon center electrophile.
中文翻译:

镍催化的炔基叠氮化物与有机硼酸的抗碳金属环化:2,3-二芳基喹啉的合成
一种抗-carbonickelative环化经由可逆alkenylnickel ë / Ž 2-叠氮基的异构化苯基炔丙基醇与芳基硼酸取得了使用酸为Ni(ACAC)2作为催化剂,以访问2,3-二芳喹啉。它代表了一个罕见的例子,即用氮中心(非碳中心的亲电试剂)捕获乙烯基金属中间体。
更新日期:2018-01-18
中文翻译:

镍催化的炔基叠氮化物与有机硼酸的抗碳金属环化:2,3-二芳基喹啉的合成
一种抗-carbonickelative环化经由可逆alkenylnickel ë / Ž 2-叠氮基的异构化苯基炔丙基醇与芳基硼酸取得了使用酸为Ni(ACAC)2作为催化剂,以访问2,3-二芳喹啉。它代表了一个罕见的例子,即用氮中心(非碳中心的亲电试剂)捕获乙烯基金属中间体。


























 京公网安备 11010802027423号
京公网安备 11010802027423号