当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modulating inflammation in a cutaneous chronic wound model by IL-10 released from collagen–silica nanocomposites via gene delivery†
Biomaterials Science ( IF 6.6 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1039/c7bm01024a Xiaolin Wang 1, 2, 3, 4, 5 , Thibaud Coradin 1, 2, 3, 4, 5 , Christophe Hélary 1, 2, 3, 4, 5
Biomaterials Science ( IF 6.6 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1039/c7bm01024a Xiaolin Wang 1, 2, 3, 4, 5 , Thibaud Coradin 1, 2, 3, 4, 5 , Christophe Hélary 1, 2, 3, 4, 5
Affiliation
|
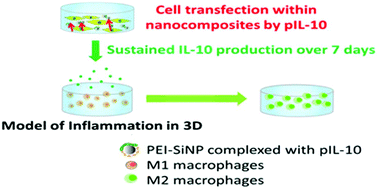
Cutaneous chronic wounds remain a major clinical challenge which requires the development of novel wound dressings. Previously, we showed that collagen–silica nanocomposites consisting of polyethyleneimine (PEI)-DNA complexes associated with silica nanoparticles (SiNP), collagen hydrogel and 3T3 fibroblasts, can work as a local “cell factory”. Indeed, the “in-gel” transfection leads to a sustained production and release of biomolecules. Herein, we further explored the possibility for nanocomposites to deliver interleukin-10 (IL-10), a potent anti-inflammatory cytokine, which favors tissue repair. Its anti-inflammatory effect was evaluated in an in vitro inflammation model carried out by LPS (lipopolysaccharide) activation of macrophages embedded in collagen gel. The IL-10 synthesis from nanocomposites was detected over one week in the range of 200–400 pg mL−1 and reached a maximum at day 5 without any observed cytotoxic effects. PEI10-SiNP outperformed free PEI10 and PEI25-SiNP, implying that the introduction of SiNP improved the transfection efficiency of low Mw of PEI. In addition, the structure and mechanical properties of collagen–silica nanocomposites were stable over one week. Subsequently, the ability of nanocomposites to modulate inflammation was tested in a 3D model of inflammation. The decrease of TNF-α and IL-1β gene expression by 20–80% indicated successful inhibition of inflammation by IL-10 released from nanocomposites. Taken together, the nanocomposites are capable of producing effective doses of IL-10 which inhibit the synthesis of pro-inflammatory cytokines and favor the expression of wound healing cytokines. Therefore, the as-constructed 3D gene delivery system represents a promising strategy for the controlled release of therapeutic biomolecules favoring cutaneous wound healing.
中文翻译:

通过基因传递 从胶原-二氧化硅纳米复合物中释放的IL-10调节皮肤慢性伤口模型中的炎症†
皮肤慢性伤口仍然是主要的临床挑战,需要开发新型伤口敷料。以前,我们证明了胶原-二氧化硅纳米复合材料由聚乙烯亚胺(PEI)-DNA复合物和二氧化硅纳米颗粒(SiNP),胶原水凝胶和3T3成纤维细胞组成,可以作为当地的“细胞工厂”。实际上,“凝胶内”转染导致生物分子的持续产生和释放。在本文中,我们进一步探讨了纳米复合材料递送白细胞介素10(IL-10)(一种有效的消炎细胞因子,有利于组织修复的可能性)的可能性。在体外评估了其抗炎作用通过LPS(脂多糖)激活嵌入胶原蛋白凝胶中的巨噬细胞进行的炎症模型。在一周内检测到了纳米复合物的IL-10合成,范围为200–400 pg mL -1,并在第5天达到最大值,而未观察到任何细胞毒性作用。PEI 10 -SiNP优于游离PEI 10和PEI 25 -SiNP,这表明SiNP的引入提高了低M w的转染效率。PEI。此外,胶原-二氧化硅纳米复合材料的结构和力学性能在一周内稳定。随后,在炎症的3D模型中测试了纳米复合材料调节炎症的能力。TNF-α和IL-1β基因表达降低20-80%,表明纳米复合物释放的IL-10成功抑制了炎症。总之,纳米复合物能够产生有效剂量的IL-10,其抑制促炎细胞因子的合成并有利于伤口愈合细胞因子的表达。因此,如此构建的3D基因传递系统代表了一种有希望的策略,可控制释放有利于皮肤伤口愈合的治疗性生物分子。
更新日期:2017-12-22
中文翻译:

通过基因传递 从胶原-二氧化硅纳米复合物中释放的IL-10调节皮肤慢性伤口模型中的炎症†
皮肤慢性伤口仍然是主要的临床挑战,需要开发新型伤口敷料。以前,我们证明了胶原-二氧化硅纳米复合材料由聚乙烯亚胺(PEI)-DNA复合物和二氧化硅纳米颗粒(SiNP),胶原水凝胶和3T3成纤维细胞组成,可以作为当地的“细胞工厂”。实际上,“凝胶内”转染导致生物分子的持续产生和释放。在本文中,我们进一步探讨了纳米复合材料递送白细胞介素10(IL-10)(一种有效的消炎细胞因子,有利于组织修复的可能性)的可能性。在体外评估了其抗炎作用通过LPS(脂多糖)激活嵌入胶原蛋白凝胶中的巨噬细胞进行的炎症模型。在一周内检测到了纳米复合物的IL-10合成,范围为200–400 pg mL -1,并在第5天达到最大值,而未观察到任何细胞毒性作用。PEI 10 -SiNP优于游离PEI 10和PEI 25 -SiNP,这表明SiNP的引入提高了低M w的转染效率。PEI。此外,胶原-二氧化硅纳米复合材料的结构和力学性能在一周内稳定。随后,在炎症的3D模型中测试了纳米复合材料调节炎症的能力。TNF-α和IL-1β基因表达降低20-80%,表明纳米复合物释放的IL-10成功抑制了炎症。总之,纳米复合物能够产生有效剂量的IL-10,其抑制促炎细胞因子的合成并有利于伤口愈合细胞因子的表达。因此,如此构建的3D基因传递系统代表了一种有希望的策略,可控制释放有利于皮肤伤口愈合的治疗性生物分子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号