Tetrahedron ( IF 2.1 ) Pub Date : 2017-12-21 , DOI: 10.1016/j.tet.2017.12.040 Ryan G Anderson 1 , Brianna M Jett 1 , Andrew McNally 1
|
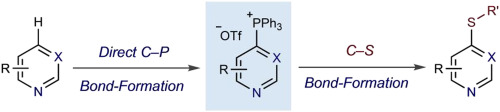
Heteroaryl thioethers, comprised of pyridines and diazines, are an important class of compounds with relevance to medicinal chemistry. Metal-catalyzed cross-couplings and SNAr reactions are traditionally used to form C–S bonds in these systems but are limited by available halogenated precursors. An alternative approach is presented where pyridines and diazines are transformed into heterocyclic phosphonium salts and then C–S bonds are formed by adding thiolate nucleophiles. The process is 4-selective for pyridines, simple to execute and can be used to make derivatives of complex pharmaceuticals.
中文翻译:

通过鏻离子偶联反应选择性形成杂芳基硫醚
杂芳基硫醚由吡啶和二嗪组成,是与药物化学相关的一类重要化合物。金属催化的交叉偶联和SN Ar反应传统上用于在这些系统中形成C-S键,但受到可用卤化前体的限制。提出了另一种方法,其中将吡啶和二嗪转化为杂环鏻盐,然后通过添加硫醇盐亲核试剂形成C-S键。该工艺对吡啶具有4-选择性,操作简单,可用于制备复杂药物的衍生物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号