当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Liquid–Liquid Equilibria Determination and Prediction for Ternary Systems of Water + 1,6-Hexanediamine + (1-Butanol or 1-Pentanol) at 298.2, 308.2, and 318.2 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1021/acs.jced.7b00678 Huanxin Li 1 , Junwei Zhang 1 , Yonghui Dou 1 , Li Xu 1 , Guoji Liu 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1021/acs.jced.7b00678 Huanxin Li 1 , Junwei Zhang 1 , Yonghui Dou 1 , Li Xu 1 , Guoji Liu 1
Affiliation
|
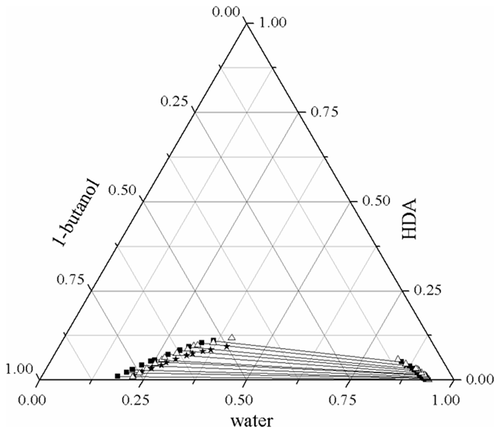
Data of liquid–liquid equilibria (LLE) for water + 1,6-hexanediamine + 1-butanol and water + 1,6-hexanediamine + 1-pentanol ternary systems were determined at T = 298.2, 308.2, and 318.2 K under atmospheric pressure. The distribution coefficients and selectivity factors calculated from LLE data were applied to evaluate the feasibility and effectiveness of extracting 1,6-hexanediamine from the aqueous solution. The results showed that the selectivity factors decrease with the amount of 1,6-hexanediamine increasing in the aqueous phase, and the effect of temperature is insignificant in this study. The nonrandom two-liquid and universal quasichemical activity coefficient models were also applied to regress the experimental equilibrium data of the two ternary systems and the root mean square deviation values between the correlated and experimental results were lower than 0.7%. It turned out that these phase equilibrium behaviors can be described by the two models. Meanwhile, the binary model parameters were acquired from the relevant process simulations.
中文翻译:

水+ 1,6-己二胺+(1-丁醇或1-戊醇)三元体系在298.2、308.2和318.2 K时的液-液平衡测定和预测
在T处测定水+ 1,6-己二胺+ 1-丁醇和水+ 1,6-己二胺+ 1-戊醇三元体系的液-液平衡(LLE)数据在大气压下= 298.2、308.2和318.2K。利用LLE数据计算出的分布系数和选择性因子,评价了从水溶液中提取1,6-己二胺的可行性和有效性。结果表明,选择性因子随水相中1,6-己二胺含量的增加而降低,而温度的影响在本研究中无关紧要。还应用了非随机的两液体和通用的准化学活性系数模型对两个三元体系的实验平衡数据进行回归,相关和实验结果之间的均方根偏差值均低于0.7%。事实证明,可以用两个模型来描述这些相平衡行为。同时,
更新日期:2017-12-21
中文翻译:

水+ 1,6-己二胺+(1-丁醇或1-戊醇)三元体系在298.2、308.2和318.2 K时的液-液平衡测定和预测
在T处测定水+ 1,6-己二胺+ 1-丁醇和水+ 1,6-己二胺+ 1-戊醇三元体系的液-液平衡(LLE)数据在大气压下= 298.2、308.2和318.2K。利用LLE数据计算出的分布系数和选择性因子,评价了从水溶液中提取1,6-己二胺的可行性和有效性。结果表明,选择性因子随水相中1,6-己二胺含量的增加而降低,而温度的影响在本研究中无关紧要。还应用了非随机的两液体和通用的准化学活性系数模型对两个三元体系的实验平衡数据进行回归,相关和实验结果之间的均方根偏差值均低于0.7%。事实证明,可以用两个模型来描述这些相平衡行为。同时,


























 京公网安备 11010802027423号
京公网安备 11010802027423号