当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phospholyl(borane) amino acids and peptides: Stereoselective synthesis and fluorescent properties with large Stokes shift.
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-09 , DOI: 10.1021/jacs.7b10954 Mathieu Arribat 1 , Emmanuelle Rémond 1 , Sébastien Clément 2 , Arie Van Der Lee 3 , Florine Cavelier 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-09 , DOI: 10.1021/jacs.7b10954 Mathieu Arribat 1 , Emmanuelle Rémond 1 , Sébastien Clément 2 , Arie Van Der Lee 3 , Florine Cavelier 1
Affiliation
|
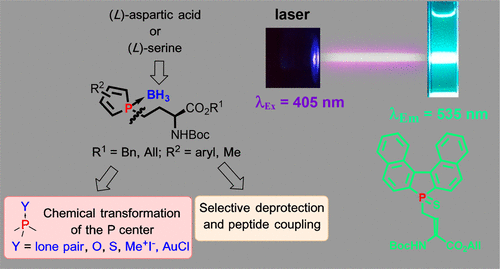
The synthesis of phospholyl(borane) amino acids was stereoselectively achieved by reaction of phospholide anion with iodo α-amino ester derived from l-aspartic acid or l-serine, followed by in situ complexation with borane. Phospholyl(borane) amino acids are easy to store and can be subjected to direct transformation into the corresponding free phospholyl, gold complex, oxide or sulfur derivatives as well as phospholinium salts, thus offering a variety of side chains. After selective deprotection of carboxylic function or amine, C- or N- peptide coupling with an alanine moiety proved the possible incorporation into peptides. Such phospholyl amino acid and peptide derivatives exhibit fluorescent properties with a large Stokes shift (160 nm) and fluorescence up to 535 nm, depending on the phosphole aromaticity and the chemical environment. These phospholyl(borane) amino acids constitute a new class of unnatural amino acids useful for structure-activities relationship studies and appear to be promising fluorophores for the development of labeled peptides.
中文翻译:

磷酸(硼烷)氨基酸和肽:具有大斯托克斯位移的立体选择性合成和荧光特性。
通过磷脂阴离子与衍生自 l-天冬氨酸或 l-丝氨酸的碘 α-氨基酯反应,然后与硼烷原位络合,立体选择性地合成磷酰(硼烷)氨基酸。磷酰(硼烷)氨基酸易于储存,可直接转化为相应的游离磷酰、金络合物、氧化物或硫衍生物以及磷盐,从而提供多种侧链。在羧基功能或胺的选择性脱保护后,C-或N-肽与丙氨酸部分偶联证明可能掺入肽中。这种磷酰氨基酸和肽衍生物表现出具有大斯托克斯位移 (160 nm) 和荧光高达 535 nm 的荧光特性,这取决于磷酰芳香性和化学环境。
更新日期:2018-01-09
中文翻译:

磷酸(硼烷)氨基酸和肽:具有大斯托克斯位移的立体选择性合成和荧光特性。
通过磷脂阴离子与衍生自 l-天冬氨酸或 l-丝氨酸的碘 α-氨基酯反应,然后与硼烷原位络合,立体选择性地合成磷酰(硼烷)氨基酸。磷酰(硼烷)氨基酸易于储存,可直接转化为相应的游离磷酰、金络合物、氧化物或硫衍生物以及磷盐,从而提供多种侧链。在羧基功能或胺的选择性脱保护后,C-或N-肽与丙氨酸部分偶联证明可能掺入肽中。这种磷酰氨基酸和肽衍生物表现出具有大斯托克斯位移 (160 nm) 和荧光高达 535 nm 的荧光特性,这取决于磷酰芳香性和化学环境。



























 京公网安备 11010802027423号
京公网安备 11010802027423号