当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Homocoupling of Phenylboronic Acid using Atomically Dispersed Gold on Carbon Catalysts: Catalyst Evolution Before Reaction
ChemCatChem ( IF 4.5 ) Pub Date : 2018-03-02 , DOI: 10.1002/cctc.201701840 Tanja E. Parmentier 1 , Simon R. Dawson 1 , Grazia Malta 1 , Li Lu 2 , Thomas E. Davies 1 , Simon A. Kondrat 1, 3 , Simon J. Freakley 1 , Christopher J. Kiely 1, 2 , Graham J. Hutchings 1
ChemCatChem ( IF 4.5 ) Pub Date : 2018-03-02 , DOI: 10.1002/cctc.201701840 Tanja E. Parmentier 1 , Simon R. Dawson 1 , Grazia Malta 1 , Li Lu 2 , Thomas E. Davies 1 , Simon A. Kondrat 1, 3 , Simon J. Freakley 1 , Christopher J. Kiely 1, 2 , Graham J. Hutchings 1
Affiliation
|
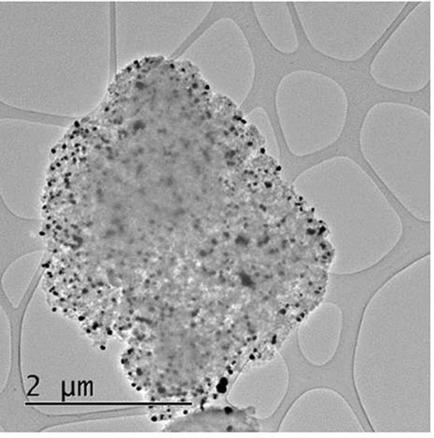
Coupling reactions to form new C−C bonds are extensively used in industrial synthetic processes. Gold has been shown to be an active catalyst for such reactions; however, conflicting reports exist as to whether cationic Au or metallic Au acts as the active species. We prepared a heterogeneous catalyst consisting of atomically dispersed Au–Clx supported on carbon and showed this to be active in the homocoupling of phenylboronic acid to biphenyl. However; characterisation of the catalyst materials, even after just a short exposure time to the reactants, revealed rapid reduction and sintering of the Au species into larger metallic nanoparticles, which we propose to be the true active species in this instance. This study suggests that if cationic Au is an active catalyst, it must be stabilised against reduction and agglomeration by either forming complexes which are more stable than common chlorides or by strongly anchoring them firmly onto alternative support materials; as in this case the carbon supported Au–Cl species were easily reduced.
中文翻译:

碳分散催化剂上原子分散金与苯基硼酸的均相偶联:反应前催化剂的演变
形成新的C-C键的偶联反应已广泛用于工业合成过程中。事实证明,金是这种反应的活性催化剂。然而,关于阳离子金还是金属金作为活性物质存在矛盾的报道。我们准备了由原子分散的Au–Cl x组成的非均相催化剂负载在碳上并显示出其在苯基硼酸与联苯的均偶联中的活性。然而; 即使仅在短时间暴露于反应物之后,催化剂材料的表征也显示出Au物种迅速还原并烧结成较大的金属纳米颗粒,在这种情况下,我们认为这是真正的活性物种。这项研究表明,如果阳离子金是一种活性催化剂,则必须通过形成比普通氯化物更稳定的络合物或将它们牢固地锚定在其他载体材料上来使其稳定,以防还原和团聚。因为在这种情况下,碳负载的Au–Cl物种很容易还原。
更新日期:2018-03-02
中文翻译:

碳分散催化剂上原子分散金与苯基硼酸的均相偶联:反应前催化剂的演变
形成新的C-C键的偶联反应已广泛用于工业合成过程中。事实证明,金是这种反应的活性催化剂。然而,关于阳离子金还是金属金作为活性物质存在矛盾的报道。我们准备了由原子分散的Au–Cl x组成的非均相催化剂负载在碳上并显示出其在苯基硼酸与联苯的均偶联中的活性。然而; 即使仅在短时间暴露于反应物之后,催化剂材料的表征也显示出Au物种迅速还原并烧结成较大的金属纳米颗粒,在这种情况下,我们认为这是真正的活性物种。这项研究表明,如果阳离子金是一种活性催化剂,则必须通过形成比普通氯化物更稳定的络合物或将它们牢固地锚定在其他载体材料上来使其稳定,以防还原和团聚。因为在这种情况下,碳负载的Au–Cl物种很容易还原。



























 京公网安备 11010802027423号
京公网安备 11010802027423号