Chem ( IF 23.5 ) Pub Date : 2017-12-21 , DOI: 10.1016/j.chempr.2017.11.007 Kamaljeet Singh , Christopher J. Fennell , Evangelos A. Coutsias , Reza Latifi , Steve Hartson , Jimmie D. Weaver
|
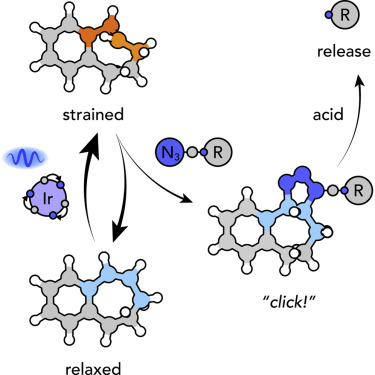
Intramolecular strain is a powerful driving force for rapid and selective chemical reactions, and it is the cornerstone of strain-induced bioconjugation. However, the use of molecules with built-in strain is often complicated as a result of instability or selectivity issues. Here, we show that such strain, and subsequent cycloadditions, can be mediated by visible light via the harvesting of photochemical energy. Through theoretical investigations and molecular engineering of strain-loadable cycloalkenes, we demonstrate the rapid chemoselective cycloaddition of alkyl azides with unstrained cycloalkenes via the transiently (reversibly) formed trans-cycloalkene. We assess this system via the rapid bioconjugation of azide-functionalized insulin. An attractive feature of this process is the cleavable nature of the linker, which makes a catch-and-release strategy possible. In broader terms, we show that conversion of photochemical energy to intramolecular ring strain is a powerful strategy that can facilitate complex chemical transformations, even in biomolecular systems.
中文翻译:

快速和选择性反应的光收集:带有应变负载烯烃的点击化学
分子内应变是快速和选择性化学反应的强大驱动力,并且是应变诱导的生物缀合的基石。然而,由于不稳定性或选择性问题,具有固有应变的分子的使用通常很复杂。在这里,我们表明这种菌株和随后的环加成反应可以通过光化学能的收获由可见光介导。通过理论研究和可应变加载的环烯烃的分子工程,我们证明了通过瞬态(可逆)形成的反式快速地将烷基叠氮化物与未应变的环烯烃进行化学选择性的环加成反应。-环烯。我们通过叠氮化物官能化胰岛素的快速生物缀合评估该系统。该过程的一个吸引人的特征是连接子的可裂解性质,这使得捕获和释放策略成为可能。从广义上讲,我们表明将光化学能转化为分子内环应变是一种强大的策略,即使在生物分子系统中,也可以促进复杂的化学转化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号