当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catechol oxidation: considerations in the design of wet adhesive materials†
Biomaterials Science ( IF 6.6 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1039/c7bm00884h Greg P. Maier 1, 2, 3, 4 , Christopher M. Bernt 1, 2, 3, 4 , Alison Butler 1, 2, 3, 4
Biomaterials Science ( IF 6.6 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1039/c7bm00884h Greg P. Maier 1, 2, 3, 4 , Christopher M. Bernt 1, 2, 3, 4 , Alison Butler 1, 2, 3, 4
Affiliation
|
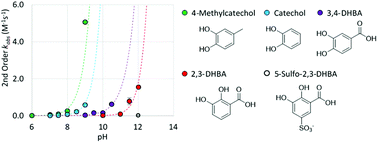
Catechols are found as functional groups in Nature as the 3,4-dihydroxy isomer, generally with electron donating substituents and as the 2,3-dihydroxy isomer, with an electron withdrawing substituent. Adhesive properties of catechol materials rely on the vicinal diol configuration, yet conversely, cohesive properties of catechol materials depend on catechol oxidation that promote subsequent crosslinking reactions. While the pH-dependent oxidation of catechol by dioxygen is well recognized, a better understanding of substituent effects on catechol redox chemistry is important in the design of new catechol-containing functional materials. The pH-dependent oxidation kinetics of catechol and substituted catechols by O2 was investigated with a Clark-type oxygen electrode. The results are consistent with a mechanism in which O2 oxidizes both mono-deprotonated and fully deprotonated catechol anions. A linear Hammett correlation for the pH-independent second order rate constants for catechol oxidation by O2 establishes that catechols functionalized with electron withdrawing groups have slower rates of oxidation by O2, whereas catechols with electron donating groups have faster rates of oxidation by O2. The Hammett correlation allows for selection of functionalized catechols with redox properties ideally suited for adhesive or crosslinking applications.
中文翻译:

邻苯二酚氧化:湿式粘合剂材料设计中的注意事项†
在自然界中发现邻苯二酚作为3,4-二羟基异构体(通常带有给电子取代基)和作为2,3-二羟基异构体(带有吸电子取代基)作为官能团。邻苯二酚材料的粘合性能取决于邻二醇的结构,但相反,邻苯二酚材料的粘合性能取决于促进后续交联反应的邻苯二酚氧化。尽管人们已经认识到双氧对邻苯二酚的pH依赖性氧化作用,但在设计新型的含有邻苯二酚的功能材料时,更好地了解取代基对邻苯二酚氧化还原化学的影响很重要。O 2对邻苯二酚和取代邻苯二酚的pH依赖性氧化动力学用Clark型氧电极进行了研究。该结果与O 2氧化单去质子化和完全去质子化的邻苯二酚阴离子的机理一致。线性的pH非依赖性的二阶速率常数儿茶酚氧化哈米特相关被O 2规定,具有吸电子基团官能化的儿茶酚具有被O氧化的速率较慢2,而具有供电子基团的儿茶酚被O具有氧化的更快的速率2。Hammett相关性允许选择具有氧化还原特性的官能化邻苯二酚,非常适合用于粘合剂或交联应用。
更新日期:2017-12-21
中文翻译:

邻苯二酚氧化:湿式粘合剂材料设计中的注意事项†
在自然界中发现邻苯二酚作为3,4-二羟基异构体(通常带有给电子取代基)和作为2,3-二羟基异构体(带有吸电子取代基)作为官能团。邻苯二酚材料的粘合性能取决于邻二醇的结构,但相反,邻苯二酚材料的粘合性能取决于促进后续交联反应的邻苯二酚氧化。尽管人们已经认识到双氧对邻苯二酚的pH依赖性氧化作用,但在设计新型的含有邻苯二酚的功能材料时,更好地了解取代基对邻苯二酚氧化还原化学的影响很重要。O 2对邻苯二酚和取代邻苯二酚的pH依赖性氧化动力学用Clark型氧电极进行了研究。该结果与O 2氧化单去质子化和完全去质子化的邻苯二酚阴离子的机理一致。线性的pH非依赖性的二阶速率常数儿茶酚氧化哈米特相关被O 2规定,具有吸电子基团官能化的儿茶酚具有被O氧化的速率较慢2,而具有供电子基团的儿茶酚被O具有氧化的更快的速率2。Hammett相关性允许选择具有氧化还原特性的官能化邻苯二酚,非常适合用于粘合剂或交联应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号