当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
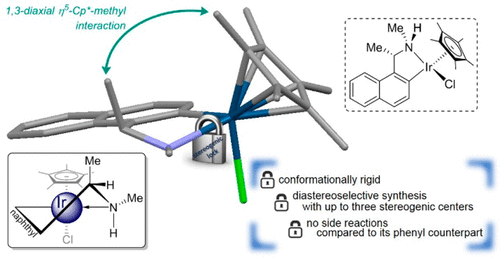
Stereogenic Lock in 1-Naphthylethanamine Complexes for Catalyst and Auxiliary Design: Structural and Reactivity Analysis for Cycloiridated Pseudotetrahedral Complexes
Organometallics ( IF 2.8 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1021/acs.organomet.7b00760 Houguang Jeremy Chen 1 , Ronald Hong Xiang Teo 1 , Yongxin Li 1 , Sumod A. Pullarkat 1 , Pak-Hing Leung 1
Organometallics ( IF 2.8 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1021/acs.organomet.7b00760 Houguang Jeremy Chen 1 , Ronald Hong Xiang Teo 1 , Yongxin Li 1 , Sumod A. Pullarkat 1 , Pak-Hing Leung 1
Affiliation
|
A series of optically active pseudo-tetrahedral five-membered cyclometalated 1-naphthylethanamine iridium(III) complexes were prepared and characterized to analyze the efficacy of the stereogenic conformational lock in both solid and solution phases. The synthesis of the iridacycles was diastereoselective, and the compounds were found to be conformationally rigid. In comparison to its phenyl derivative, the structural lock prevented oxidation of the amine moiety within the five-membered organometallic ring during its synthesis. With up to three stereogenic centers in one of the naphthalene complexes, the stereochemistry of the metallacycle remained stable to both thermal and chemical changes. In terms of catalytic performance, the complexes displayed excellent activity for the asymmetric hydrogen transfer reaction, albeit with modest enantioselectivities.
中文翻译:

1-萘酞胺配合物的立体锁定用于催化剂和辅助设计:环化伪四面体配合物的结构和反应性分析
制备了一系列光学活性的拟四面体五元环金属化的1-萘基胺铱(III)配合物,并对其进行了表征,以分析固相和溶液相中立体构象锁的功效。iridacycles的合成是非对映选择性的,并且发现这些化合物在构象上是刚性的。与它的苯基衍生物相比,结构锁防止了五元有机金属环中胺部分在合成过程中的氧化。在一种萘配合物中最多有三个立体异构中心,金属环的立体化学对热和化学变化均保持稳定。在催化性能方面,络合物对不对称氢转移反应显示出优异的活性,
更新日期:2017-12-21
中文翻译:

1-萘酞胺配合物的立体锁定用于催化剂和辅助设计:环化伪四面体配合物的结构和反应性分析
制备了一系列光学活性的拟四面体五元环金属化的1-萘基胺铱(III)配合物,并对其进行了表征,以分析固相和溶液相中立体构象锁的功效。iridacycles的合成是非对映选择性的,并且发现这些化合物在构象上是刚性的。与它的苯基衍生物相比,结构锁防止了五元有机金属环中胺部分在合成过程中的氧化。在一种萘配合物中最多有三个立体异构中心,金属环的立体化学对热和化学变化均保持稳定。在催化性能方面,络合物对不对称氢转移反应显示出优异的活性,



























 京公网安备 11010802027423号
京公网安备 11010802027423号