当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism of OmpG pH-dependent gating from loop ensemble and single channel studies
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-11 , DOI: 10.1021/jacs.7b11979 Alan Perez-Rathke 1 , Monifa A. Fahie , Christina Chisholm , Jie Liang 1 , Min Chen
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-11 , DOI: 10.1021/jacs.7b11979 Alan Perez-Rathke 1 , Monifa A. Fahie , Christina Chisholm , Jie Liang 1 , Min Chen
Affiliation
|
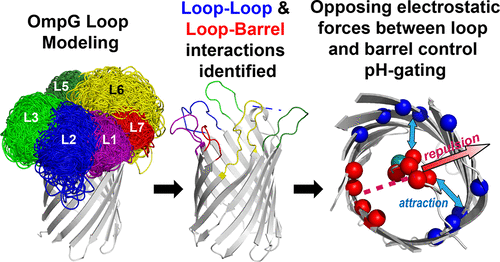
Outer membrane protein G (OmpG) from Escherichia coli has exhibited pH-dependent gating that can be employed by bacteria to alter the permeability of their outer membranes in response to environmental changes. We developed a computational model, Protein Topology of Zoetic Loops (Pretzel), to investigate the roles of OmpG extracellular loops implicated in gating. The key interactions predicted by our model were verified by single-channel recording data. Our results indicate that the gating equilibrium is primarily controlled by an electrostatic interaction network formed between the gating loop and charged residues in the lumen. The results shed light on the mechanism of OmpG gating and will provide a fundamental basis for the engineering of OmpG as a nanopore sensor. Our computational Pretzel model could be applied to other outer membrane proteins that contain intricate dynamic loops that are functionally important.
中文翻译:

来自循环集成和单通道研究的 OmpG pH 依赖性门控机制
来自大肠杆菌的外膜蛋白 G (OmpG) 表现出 pH 依赖性门控,细菌可以利用该门控来改变其外膜的渗透性以响应环境变化。我们开发了一个计算模型,动物环蛋白拓扑 (Pretzel),以研究 OmpG 细胞外环在门控中的作用。我们的模型预测的关键相互作用通过单通道记录数据进行了验证。我们的结果表明,门控平衡主要由门控回路和管腔中带电残留物之间形成的静电相互作用网络控制。该结果阐明了 OmpG 门控的机制,并将为 OmpG 作为纳米孔传感器的工程设计提供基本依据。
更新日期:2018-01-11
中文翻译:

来自循环集成和单通道研究的 OmpG pH 依赖性门控机制
来自大肠杆菌的外膜蛋白 G (OmpG) 表现出 pH 依赖性门控,细菌可以利用该门控来改变其外膜的渗透性以响应环境变化。我们开发了一个计算模型,动物环蛋白拓扑 (Pretzel),以研究 OmpG 细胞外环在门控中的作用。我们的模型预测的关键相互作用通过单通道记录数据进行了验证。我们的结果表明,门控平衡主要由门控回路和管腔中带电残留物之间形成的静电相互作用网络控制。该结果阐明了 OmpG 门控的机制,并将为 OmpG 作为纳米孔传感器的工程设计提供基本依据。


























 京公网安备 11010802027423号
京公网安备 11010802027423号